Floating Bowling Balls
We all know that certain things float in water while other things sink, but why? Do all heavy things sink? Why does a penny sink […]
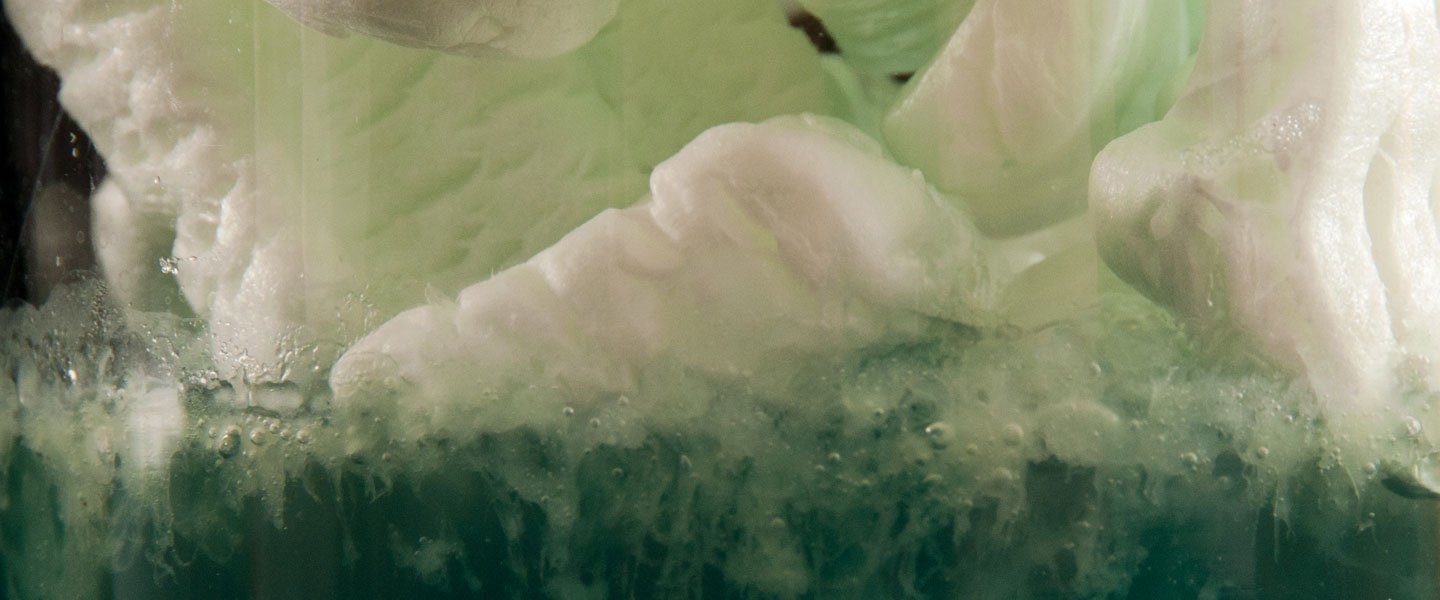
They seem to magically “disappear.” In fact, the Styrofoam reacts with the solvent to reveal the fact that Styrofoam is made up of long strands of styrene molecules with lots of air pockets. This demonstration also reminds us about the importance of reducing our use of Styrofoam and replacing it with more Earth-friendly packing materials.
Fill two bowls one-half full with water.

Put a handful of Styrofoam peanuts in one bowl and a handful of starch-based peanuts in the other bowl.

Notice how the Styrofoam does not dissolve.

How to Make Styrofoam Packing Peanuts Vanish
Fill a beaker with 60 milliliters (that’s about 2 oz) of acetone.

Drop Styrofoam peanuts into the beaker and watch what happens. It looks like they’re melting, but they’re really just dissolving (melting requires heat). You’ll be amazed to see how much Styrofoam will dissolve in just a few ounces of acetone.

If you have a large enough beaker, try placing a Styrofoam cup into the beaker to see what happens. With just a little swirl, the entire cup will vanish in seconds.

WARNING! TEACHERS ONLY!
This demonstration is provided for educational purposes only and should not be attempted if you are not a properly trained professional. Please follow all of the manufacturer’s safety precautions listed on the container of acetone. This solvent is very flammable. Keep away from all flames.
Place a Styrofoam head used to display wigs on the table. The next step is the trickiest one—to carve a huge hole in the top of the Styrofoam head. You want the hole big enough to hold the 250-milliliter beaker. For those nonmetric people, you’ll want a glass that holds about 8 ounces of liquid. You can use an electric drill with a doorknob hole cutter blade to get the hole started, but it’s going to take a little patience until the hole is just the right size.

Fill the beaker with 200 milliliters of acetone (about 6 oz) and carefully lower the beaker into the hole. Be careful not to spill any acetone on the Styrofoam head or it too will dissolve! You might also want to put safety glasses on the Styrofoam head, just for good measure.

You’ll need a sharp knife to cut the Styrofoam board into long strips. The width of each strip is determined by the diameter of the glass container in the head (250-mL beaker or otherwise). Cut as many strips as you feel the urge to make disappear. NOTE: Some Styrofoam board material has a thin, plastic covering on both sides. Remove any plastic wrapping before doing the demo.

Use a Sharpie pen to write down anything important you want the “head” to know. Consider writing the three Rs of recycling on the three strips—REDUCE, REUSE, and RECYCLE. This would be a great activity to do as a review for a test to “cram” the most important information into the Styrofoam head and your students’ heads.

It’s showtime! The story line is up to you, so be creative. When it’s time to make the strip vanish, slowly push the strip into the beaker of acetone, being careful not to make the acetone erupt onto the Styrofoam head. The illusion is great, as it looks like knowledge is being shoved piece by piece into the head.
Styrofoam is actually a common name for a material called polystyrene, which is a polymer made up of a long chain of molecules. The packing “peanuts” are made in a special process whereby air is blown into liquid polystyrene to make puffy peanut-shaped pieces out of a very tiny quantity of polystyrene. If you break apart a Styrofoam peanut, you’ll see little pockets of air that serve as a cushion to protect delicate items that are packed and shipped. The acetone is a solvent that easily breaks down the polystyrene, releasing the little air pockets and leaving very little residue at the end. In other words, the polystyrene dissolves in the acetone.
As a science teacher, science enthusiast, or an environ- mentalist, you are aware of the bad effects that Styrofoam has on our environment. That’s why many companies have turned to starch packing peanuts as a substitute for Styrofoam. Instead of taking up space in the landfills, starch peanuts dissolve in water to make landfill gravy! So why not just use acetone to dissolve waste polystyrene? Problem solved, right? Not quite, since the acetone presents its own environmental and energy consumption issues.
Here’s a fun activity for teachers. Engage students in a peanut race by seeing which team can fill a bucket first with polystyrene peanuts. Of course, one bucket will secretly contain acetone and the team with this bucket hasn’t got a prayer of winning! Use extreme care when handling acetone. Follow the manufacturer’s directions for proper use and disposal.
Colored Starch Noodles
Several companies have turned starch peanuts into an arts and crafts project for kids by sim- ply adding color to starch peanuts and calling them “building noodles.” Here’s how it works: simply wet one end of the colored starch peanut with a dab of water and stick it to another peanut. Build houses, hats, glasses, letters, people, a medieval castle with flying buttresses . . . just build anything! Use the colored starch peanuts as an icebreaker or team-building activity with adults or kids. They’re great for staff meetings!
WARNING! TEACHERS ONLY!
This demonstration is provided for educational purposes only and should not be attempted if you are not a properly trained professional. Please follow all of the manufacturer’s safety precautions listed on the container of acetone. This solvent is very flammable. Keep away from all flames.
You Have a Choice
Currently about 200 million cubic feet per year of polystyrene “loose fill” (packaging material) is used in the United States. Although some companies try to reuse the packing material, most of the polystyrene loose fill is disposed of in a landfill. As students of science, we need to carefully examine such products and ask these questions: How is the material made, and what happens to it after it is used? One of the properties of polystyrene loose fill is that it does not compress easily. While this is beneficial when trying to protect something from being crushed or broken, it poses a problem when trying to dispose of it in a landfill. As a result, environmentally conscious companies sought a solution to these problems. One such solution is called Eco-Foam loose fill. It provides the ease of use and cushioning of polystyrene, but it gives many other reuse or disposal options for the future. It readily decomposes in water and can be reused for your own packages, or you can dispose of it by putting it in your compost pile, watering it into your lawn, or washing it down the sink.
Eco-Foam is made almost entirely from an annually renewable resource . . . corn! The remaining ingredient is a water-soluble organic polymer called “polyvinyl alcohol.” This organic polymer is made from carbon, hydrogen, and oxygen—the building blocks of life. When polyvinyl alcohol is exposed to water, naturally occurring bacteria feed on this organic polymer. Under wet conditions, the bacteria will use the starch (which is also composed of carbon, hydrogen, and oxygen) and polyvinyl alcohol as food to begin the cycle of life again.
Many people feel that the answer to our solid waste problem is recycling. While this method will go a long way to help our solid waste problems, it is not the whole solution. One good suggestion is to use as little of the material as possible. Secondly, it makes sense to use a natural product (instead of a synthetic product) that will break down when we are finished using it. We must remember how to reuse!