Ultimate Can Crusher - 55 Gallon Drum
Steve Spangler often says… “Anything worth doing is worth overdoing.” That’s probably what happened here when he tried to super-size the classic Can Crusher experiment […]
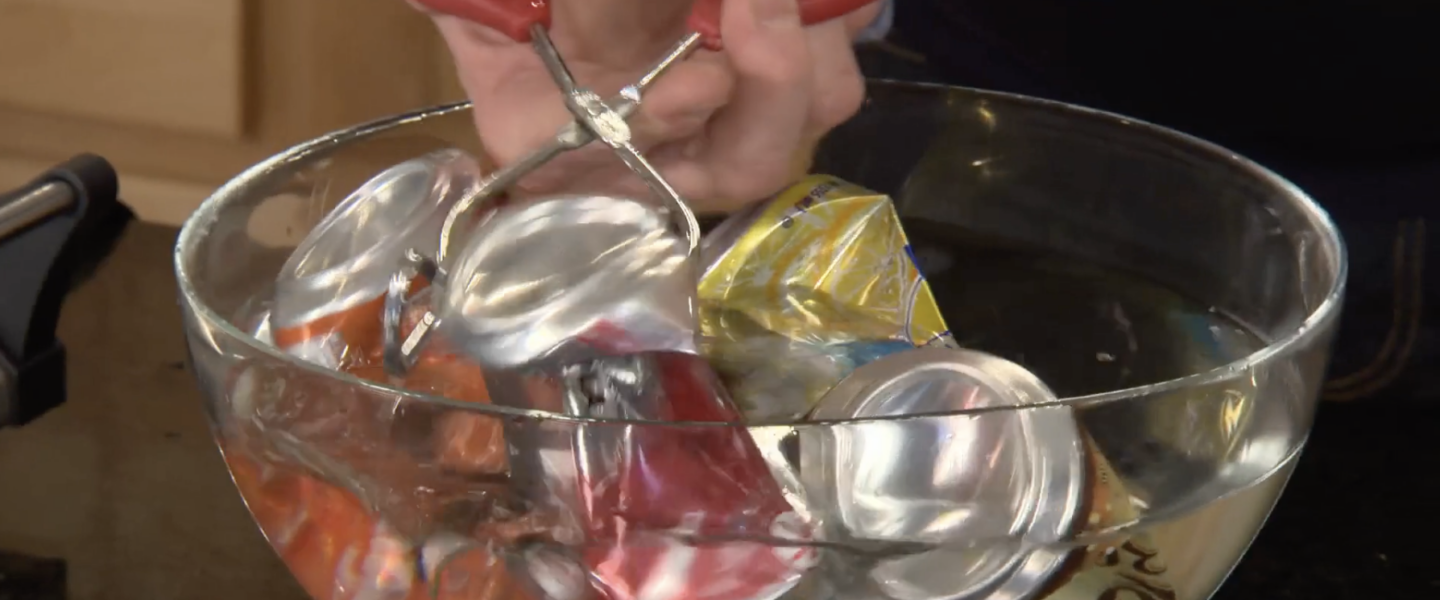
You could use your foot, your hands, or even your head (not advised) to crush a soda can. But nothing compares to the fun you’ll have doing the soda can implosion experiment. Just wait until the can goes “POP!” Then you’ll see who has nerves of steel.

Start by rinsing out the soda cans to remove any leftover soda goo.

Fill the bowl with cold water (the colder the better).

Add 1 generous tablespoon of water to the empty soda can (just enough to cover the bottom of the can).

Place the can directly on the burner of the stove while it is in the “OFF” position. It’s time for that adult to turn on the burner to heat the water. Soon you’ll hear the bubbling sound of the water boiling and you’ll see the water vapor rising out from the can. Continue heating the can for one more minute.

It’s important to think through this next part before you do it. Here’s what’s going to happen: you’re going to use the tongs to lift the can off the burner, turn it upside down, and plunge the mouth of the can down into the bowl of water. Get a good grip on the can near its bottom with the tongs, and hold the tongs so that your hand is in the palm up position. Using one swift motion, lift the can off the burner, turn it upside down, and plunge it into the cold water. Don’t hesitate . . . just do it!

Wow, and you thought that you had nerves of steel. The can literally imploded. Before you jump ahead to the explanation, stop to ponder how this works. What force is great enough to crush the can?

Don’t just sit there . . . get back to that stove and do it again! Each time you repeat the experiment, carefully observe what is happening in order to try to figure out what’s going on.
Here’s the real scoop on the science of the imploding can. Before heating, the can is filled with water and air. By boiling the water, the water changes states from a liquid to a gas. This gas is called water vapor. The water vapor pushes the air that was originally inside the can out into the atmosphere. When the can is turned upside down and placed in the water, the mouth of the can forms an airtight seal against the surface of the water in the bowl. In just a split second, all of the water vapor that pushed the air out of the can and filled up the inside of the can turns into only a drop or two of liquid, which takes up much less space. This small amount of condensed water cannot exert much pressure on the inside walls of the can, and none of the outside air can get back into the can. The result is the pressure of the air pushing from the outside of the can is great enough to crush it.
The sudden collapsing of an object toward its center is called an implosion. Nature wants things to be in a state of equilibrium or balance. To make the internal pressure of the can balance with the external pressure on the can, the can implodes. That’s right, air pressure is powerful!
One more thing . . . you probably noticed that the can was filled with water after it imploded. This is a great illustration of how air is pushing all around us. Specifically, the outside air pressure was pushing downward on the surface of the water. Since the air pressure inside the can was less than the pressure outside the can, water from the bowl was literally pushed up and into the can.
This action is similar to what happens when you drink from a straw. Though we say we are “sucking” liquid up through the straw, we really aren’t. To put it simply, science doesn’t suck . . . it just pushes and pulls. Outside air pressure is pushing down on the surface of the liquid. When you reduce the pressure in your mouth (that sucking action) the outside pressure is greater than the pressure inside your mouth and the soda shoots up through the straw and into your mouth. The same thing is true with the can. The outside air pressure pushing downward on the surface of the water is greater than the force inside the can and the water gets pushed up into the can.
WARNING! IMPORTANT SAFETY RULES
This experiment requires the use of a burner on a stove to heat some water. Children should not perform this experiment without adult supervision. Adults shouldn’t do it either unless a really smart kid is watching over their shoulder.