Mentos Super Soda Dispenser 3000
Steve’s son, Jack Spangler, decided that it is just too hard to pour soft drinks by turning a 2-liter bottle of soda upside down. The […]
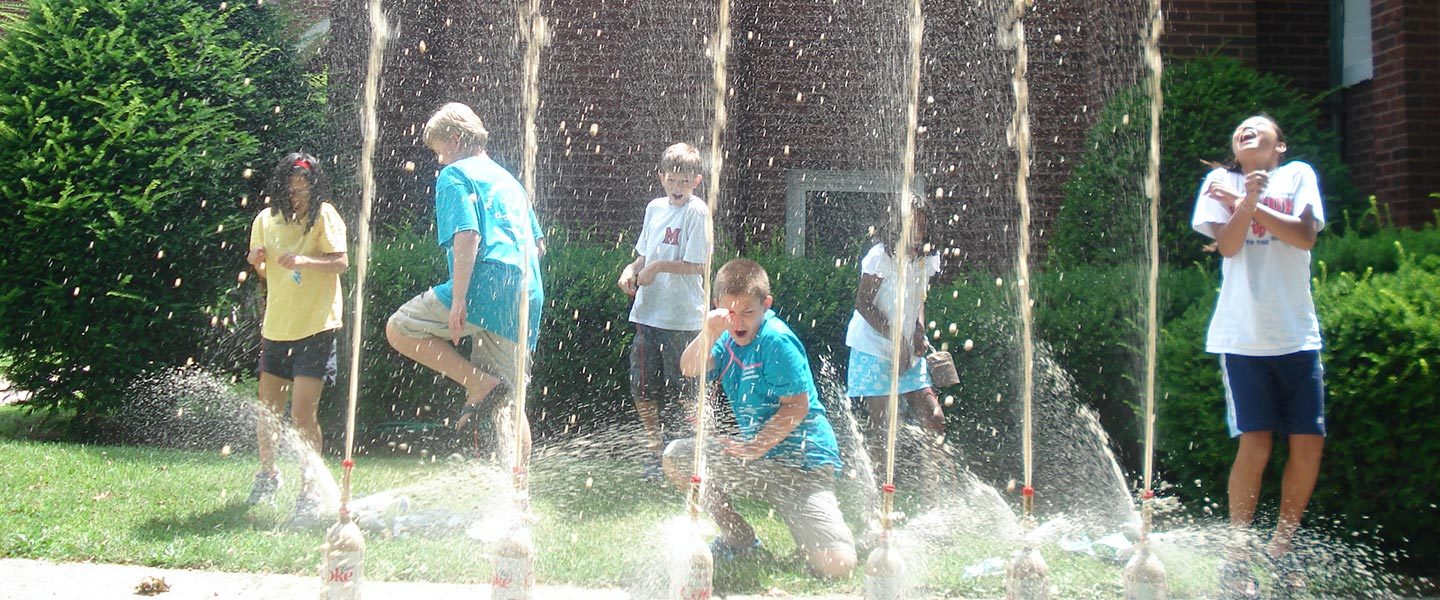
It’s been called the “vinegar and baking soda” reaction for a new generation. While science teachers have been dropping candies and mints into 2-liter bottles of soda for years to release all of the dissolved carbon dioxide, the Mentos and Diet Coke reaction became world-famous in 2005. Fueled by hundreds of blogs and popular online sharing sites like youtube, this once obscure reaction became an Internet sensation. Once you get past the initial gee-whiz factor, there’s some amazing science behind a carbonated beverage and a chewy mint.
This full version of this experiment appears in Steve Spangler’s book called Naked Eggs and Flying Potatoes – Unforgettable Experiments That Make Science Fun

This activity is probably best done outside in the middle of an abandoned field or on a huge lawn.

Carefully open the bottle of diet soda. Again, the choice of diet over regular soda is purely a preference based on the fact that erupting regular soda becomes a sticky mess to clean up because it contains sugar. Diet soda uses artificial sweeteners instead of sugar, and consequently, it’s not sticky. Later on in the experiment, you’ll be invited to compare the geyser power of diet versus regular soda, but for now we’ll start with a 2-liter bottle of diet soda.

Position the bottle on the ground so that it will not tip over.

Let’s start with seven Mentos for our first attempt. The goal is to drop all seven Mentos into the bottle of soda at the same time (which is trickier than you might think). One method for doing this is to roll a piece of paper into a tube just big enough to hold the loose Mentos. Other methods include using a large plastic test tube to hold the Mentos or using my Geyser Tube toy invention, which was created to solve this very problem. Assuming that you’re using the paper tube method, you’ll want to load the seven Mentos into the tube, cover the bottom of the tube with your finger, and position the tube directly over the mouth of the bottle. When you pull your finger out of the way, all seven Mentos should fall into the bottle at the same time.

Enough waiting . . . this anticipation is killing me. 3-2-1 drop the Mentos!

This final step is very important . . . run away! But don’t forget to look back at the amazing eruption of soda.

If spectators were watching your exploits, someone is bound to yell out, “Do it again!” and that’s exactly what you’re going to do.
Why do Mentos turn ordinary bottles of diet soda into geysers of fun? The answer is a little more complicated than you might think. Let’s start with the soda . . .
Soda pop is made of sugar or artificial sweetener, flavoring, water, and preservatives. The thing that makes soda bubbly is invisible carbon dioxide (CO2), which is pumped into bottles at the bottling factory using lots of pressure. If you shake a bottle or can of soda, some of the gas comes out of the solution and the bubbles cling to the inside walls of the container (thanks to tiny pits and imperfections on the inside surface of the bottle called nucleation sites). When you open the container, the bubbles quickly rise to the top pushing the liquid out of the way. In other words, the liquid sprays everywhere.
DOWNLOAD THE FULL MENTOS AND DIET COKE EXPERIMENT
Is there another way for the CO2 to escape? Try this. Drop an object like a raisin or a piece of uncooked pasta into a glass of soda and notice how bubbles immediately form on the surface of the object. These are CO2 bubbles leaving the soda and attaching themselves to the object. For example, adding salt to soda causes it to foam up because thousands of little bubbles form on the surface of each grain of salt. This bubbling process is called nucleation, and the places where the bubbles form, whether on the sides of the can, on an object, or around a tiny grain of salt, are the nucleation sites.
Why are Mentos so Special?
The reason why Mentos work so well is twofold—tiny pits on the surface of the mint, and the weight of the Mentos itself. Each Mentos mint has thousands of tiny pits all over the surface. These tiny pits act as nucleation sites—perfect places for CO2 bubbles to form. As soon as the Mentos hit the soda, bubbles form all over the surfaces of the candies and then quickly rise to the surface of the liquid. Couple this with the fact that the Mentos candies are heavy and sink to the bottom of the bottle and you’ve got a double whammy. The gas released by the Mentos literally pushes all of the liquid up and out of the bottle in an incredible soda blast.
Measuring the Height of the Geyser
To make any of these tests meaningful, you need to find a way to measure the height of the eruption. A friend or parent with a video camera is a great way to watch and document the results of your experiment, but you’ll also need some specific measurements or data. Try placing the soda bottle next to the wall of a brick building (after getting permission from the building’s owner). Measure the height of the geyser by counting the number of bricks that are wet once the geyser stops. If you want a more specific measurement, use chalk to mark off 1-foot increments on the brick wall before you drop the Mentos into the bottle of soda. Make comparisons, create a chart with your data, and draw some conclusions. Be sure to thank the building’s owner and to hose off the wall of the building when you are finished!
Measuring the Volume of the Geyser
If you want to examine the volume of the geyser instead of the height, make note of the volume of a full bottle of soda before you drop the Mentos into it. (Okay, it’s a trick question because a 2-liter bottle of soda holds . . . 2 liters!) Once the geyser stops, pour out the remaining contents of the bottle and measure how much liquid is left. You could use a beaker or a graduated cylinder to measure the remaining liquid in milliliters. Remember that 1 liter is equivalent to 1000 mL. Subtract the remaining amount of liquid from the original volume of the bottle to calculate the volume of the geyser. Then make comparisons, create a chart with your data, and draw some conclusions.
How Many Mentos Work Best?
This has to be the number one question everyone asks about this experiment. What is the best number of Mentos to use to make the highest-shooting geyser? This is a great topic for a science project—you’ll need lots of soda and Mentos, and a few friends to help record all of the data.
Be sure that the soda bottles are all the same brand and type. It’s also important that all of the test bottles are stored in the same place so that the liquid in each bottle is the same temperature.
Line up a row of ten 2-liter bottles against a brick wall (see “Measuring the Height of the Geyser”). Each bottle will receive a different number of Mentos. Drop one Mentos into the first bottle and record the height by counting the wet bricks (or set up your own scale behind each soda bottle). Drop two Mentos into the second bottle, and so on until you’ve completed all ten bottles.
Of course, this could go on forever, but you’ll start to see a trend in your data that shows the maximum height of the geyser for a certain number of Mentos. Many soda geyser-ologists believe that seven Mentos produce the highest-shooting geyser. Using any more than seven Mentos is just a waste, according to these soda-soaked science enthusiasts. What do your results reveal about the effect of the number of Mentos on the height of the geyser?
The Brand Test
You guessed it . . . it’s time to put your favorite soda to the test. Does one brand produce higher-flying geysers? How does generic soda stack up against the big name brands? If you’re doing a science fair project, your initial question might be, “What is the effect of the brand of soda on the height of the geyser?”
Use your data from the previous test to determine the standard number of Mentos to use for this test. The only variable you’ll change in this test is the brand of soda while everything else remains the same (the number of Mentos and the amount of soda). Again, make sure all of the soda is at the same temperature because temperature plays an important role in the reaction. The brand of soda is the only thing that changes (the variable).
Just think . . . your results could help determine the next Mentos Geyser craze!
The Temperature Test
What is the effect of temperature on the height of the geyser? Does warm soda shoot up higher than cold soda? The key is to keep every launch fair and to make sure the only variable is the temperature of the soda. You’ll need a thermometer to record the temperature of the soda just before you launch it.
To enforce the fairness factor, you must stick with one brand of soda for the entire test. Let’s use Diet Coke in this example. You’ll want to purchase three bottles of Diet Coke and two rolls of Mentos. You’re going to set up three tests—warm soda, room temperature soda, and cold soda. Place one bottle of Diet Coke in the refrigerator and let it sit overnight. Place the second bottle in a place where it can reach room temperature overnight. There are two safe ways to warm the other bottle of soda. The simplest method is to let the unopened bottle sit in the sun for several hours. You can also place the bottle of unopened soda in a bucket of warm water. Never use a stove or microwave to heat a bottle of soda.
It’s time to return to your launching site. Check to make sure your measuring scale is in place (counting bricks or using an alternative scale against the wall). Let’s start with the bottle of cold Diet Coke. Open the bottle and dip the thermometer down into the soda. Record the temperature. Load seven Mentos into your paper roll and drop them into the soda. Immediately record the data for the cold soda test. Repeat the same procedure for the bottle of soda at room temperature and for the bottle of warm soda. It’s important to use the same number of Mentos for each test and to drop them the same way.
No matter which brand of soda you tested, the warm bottle probably produced the highest-shooting geyser. Warm soda tends to fizz much more than cold soda. Why? The answer lies in the solubility of gases in liquids. The warmer the liquid, the less gas can be dissolved in that liquid. The colder the liquid, the more gas can be dissolved in that liquid. This is because as the liquid is heated, the gas within that liquid is also heated, causing the gas molecules to move faster and faster. As the molecules move faster, they diffuse out of the liquid, leaving less gas dissolved in that liquid. In colder liquids the gas molecules move very slowly, causing them to diffuse out of the solution much more slowly. More gas tends to stay in solution when the liquid is cold. This is why at the bottling plant CO2 is pumped into the cans or bottles when the fluid is just above freezing—around 35 degrees Fahrenheit. This low temperature allows the maximum amount of CO2 to dissolve in the soda, keeping the carbonation levels as high as possible.
Simply dropping Mentos into a bottle of soda to make a geyser isn’t really science—it’s just a fun trick to do in the backyard. The real learning takes place when you start to change one variable at a time to see how it affects the performance of the geyser.
PURCHASE Steve Spangler Original Mentos Geyser Tube on Amazon
The Big Blast
After completing all of these tests, you’ve become somewhat of a Mentos Geyser expert who has the research to support the answer to the question, “How can you make the highest-shooting Mentos geyser?” Each test isolated an independent variable, and combining all of the information you discovered into one launch is a great way to wrap up your science fair project. For example, based on your individual test results, you might have arrived at this recipe for the best Mentos Geyser:
By using the scientific method and some critical thinking skills, you’ve successfully turned a great gee-whiz science trick into a research-based science fair project.
You might ask yourself, “Can I use the Mentos Geyser for my science fair project?” The answer is YES, but you’ll need to learn how to turn a cool science activity into a real science experiment. The secret is to turn your attention away from the spraying soda and concentrate on setting up an experiment where you isolate a single variable and observe the results.
To get the best results in a science experiment, you need to standardize the test conditions as much as possible. The biggest challenge in the Mentos Geyser experiment is finding a consistent way to drop the Mentos into the soda every time. The original reason I invented the Geyser Tube toy was to find a way to standardize the actual drop of the Mentos. If you’re not using the Geyser Tube, make sure to come up with your own method for dropping the Mentos into the soda the same way each time.
This full version of this experiment appears in Steve Spangler’s book called Naked Eggs and Flying Potatoes – Unforgettable Experiments That Make Science Fun
As strange as it might sound, the Mentos Geyser never actually started out using Mentos chewy mints. This science demonstration was popular among chemistry teachers back in the 1980s using a roll of Wintergreen LifeSavers and a pipe cleaner. Teachers threaded the roll of Wintergreen LifeSavers onto a pipe cleaner as an easy way to drop all of the LifeSavers into the soda at the same time. Within seconds of dropping the candies into the soda, a huge geyser would erupt from the bottle.
However, by the end of the 1990s, the manufacturer of Wintergreen LifeSavers increased the size of the mints (no one was ever certain why this happened), making the diameter of the candy too large to fit into the mouth of the soda bottle. Science teachers started experimenting (as they like to do) with other candies and mints that would have the same effect when dropped into a bottle of soda. As luck would have it, the solution to the problem was within arm’s reach of the Wintergreen LifeSavers in the candy aisle—it was Mentos chewy mints.
Because Mentos mints didn’t have holes in the middle like LifeSavers, getting them into the bottle was tricky. Everyone found their own method of quickly dropping the Mentos into the soda. Some people fashioned a tube out of paper while others used a piece of plastic tubing to load the Mentos. At the time, my solution was to load the Mentos candies into something called a Baby Soda Bottle—a test tube–like container that held an entire roll of Mentos perfectly. Oddly enough, this container was actually a “pre-form” or 2-liter soda bottle before it was blown up into a big bottle. That’s why it’s called a Baby Soda Bottle.
However, I must admit that even with the Baby Soda Bottle method, the results were not very consistent and it was challenging to get away from the bottle before it exploded. So, I solicited help from our creative team at Steve Spangler Science to come up with a Geyser Tube— a better, more consistent way to drop the Mentos into the bottle. Better yet, if we could trigger the drop of the Mentos from a distance, we wouldn’t get as wet.
The next few months were spent building trigger devices ranging from plastic tubes with sliding doors to magnets that held metal stoppers in place to an elaborate battery-operated switch that was triggered by a motion detector. We even played with ways of using the Geyser Tube to trigger multiple soda geysers in a method similar to a Rube Goldberg machine. But the bottom line was that we needed to find a way to standardize the drop of the Mentos.
As they say, the simplest design usually turns out to be the best and most elegant solution to the problem. The winning Geyser Tube design was a clear plastic tube with a special fitting that twisted onto any soda bottle. The trigger pin at the bottom of the tube prevented the Mentos from falling into the bottle until you pulled the string attached to the pin. The moment the pin was pulled, a slider ring resting above the pin fell into place and covered the holes where the trigger pin once was, and the Mentos dropped into the soda. But there was one added bonus . . . the restricted hole at the top of the plastic tube helped to build up more pressure in the bottle and launched the soda 30 feet into the air.
Fortunately, the maker of Mentos (Perfetti Van Melle) also liked the design, and we launched the Mentos Geyser Tube toy at the New York Toy Fair in February 2007. The Geyser Tube toy is currently available in toy stores and mass-market retailers throughout the country thanks to our distributor, Be Amazing Toys!
The Mentos Geyser became one of my featured demonstrations both on television and during my live stage presentations. While I had performed variations of the Mentos Geyser experiment on television many times from 2001 to 2004, my performance of the demo in September of 2005 in the backyard of NBC affiliate KUSA-TV in Denver proved to be the tipping point as the demo went from relative obscurity to Internet sensation.
My cohost for the KUSA-TV science segment was the lovely Kim Christiansen. During the commercial break, I told Kim what was going to happen and reminded her to pull her hand out of the way of the erupting geyser and to run backward. Unfortunately, Kim got so caught up in the fun that she forgot to do both . . . and got soaked in Diet Coke on live television. To add insult to injury, she did it two more times, each time getting covered in more soda, until her once pink dress was more Coke-colored than pink.
KUSA-TV News posted that original video on their website along with my blog post titled, “News Anchor Gets Soaked!” Within a few weeks, links to the video and my blog entry numbered in the thousands. I also posted the video on a new online video sharing site called YouTube (YouTube was only 7 months old at the time), and as they say, the rest is history. Within the next 12 months, over 800 Mentos Geyser-related videos were posted on YouTube, making the demo one of the most popular pop-culture science experiments in recent history.
You know the Mentos Geyser is a popular experiment when a producer from ABC’s Who Wants to Be a Millionaire calls for help writing a question. Here’s the question we came up with:
In an experiment popularized online, what candy creates an explosive geyser when dropped into a 2-liter Diet Coke bottle?
A) Skittles
B) Mint Mentos
C) Atomic Fireballs
D) Lemon Heads
The question was asked on a special College Week episode of Who Wants to Be a Millionaire. The participant got it right for $8,000, saying: “I saw it on TV and I bought Mentos and a 2-liter bottle of Diet Coke . . . so I’m going to go with Mentos. That’s my final answer.” The contestant ended up doing really well, going all the way to the $250,000 question, but he walked away with $125,000.