Dancing Spaghetti
Who taught these noodles to dance? Go on – gather up some pasta noodles, turn up the music, and get ready for an old-fashioned pasta […]
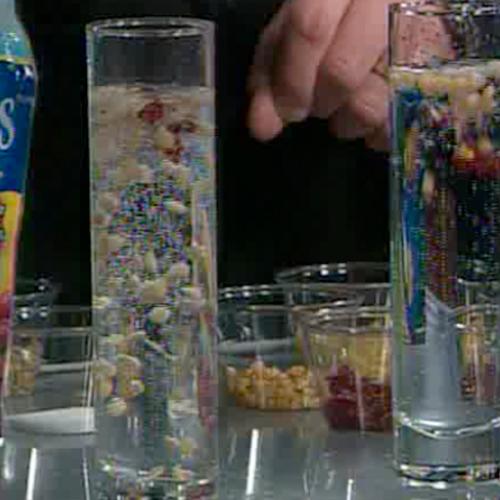
Who taught these raisins to dance, anyway? Go on – gather up some raisins, turn up the music, and get ready for a raisin romp. Just when you thought you were done at the dinner table… here is some kitchen chemistry that you can eat!
Dancing Raisins Variation
You can guess why this is an extremely popular activity among elementary teachers. We recommend that you play some dance music and encourage the kids to join in with the raisins while you learn about the science behind the dance.
The raisins will bob up and down for several minutes. This “raisin dance” is captivating to watch. Since the surface of the raisins is rough, tiny bubbles of carbon dioxide gas are attracted to it. These bubbles increase the volume of the raisin substantially, but contribute very little to its mass. As a result, the overall density of the raisin is lowered, causing it to be carried upward by the more dense fluid surrounding it.
Archimedes’ Principle states that the buoyant force exerted on a fluid is equal to the weight of fluid displaced. Since the raisins now have a greater volume, they displace more water, causing the fluid to exert a greater buoyant force. The buoyant force of the surrounding fluid is what pushes the raisins to the top.
Once the raisins reach the top, the bubbles pop upon exposure to the air. This makes the raisins more dense, causing them to sink. As more bubbles adhere to the raisins, the density of the raisins decreases and they rise to the surface again. This experiment very clearly shows that an increase in volume (as long as the mass increase is negligible) will lead to a decrease in density. The bubbles that attach themselves to the raisins are like little life jackets that make the raisins more buoyant by increasing their volume.
If you put the raisins directly in the bottle and replace the cap, eventually the raisins will stop dancing. This is because carbon dioxide gas is prevented from leaving the bottle. As a result, pressure builds up in the space above the fluid. This pressure is transmitted throughout the fluid, and the bubbles cannot grow as large. The volume of the raisins cannot increase enough to lower their density to the point where they will rise. When the cap of the bottle is removed, the bubbles grow larger, and the raisins resume the cycle of their “dance.”
The same scientific principles are at work when a child uses a set of inflated “floaties” or an inner tube at the pool. The volume of the floaties increases the child’s volume considerably. The mass of the floaties, however, is very small. The overall effect is to lower the density of the child wearing the floaties to less than that of the pool water, so that the child can float. Deflating the floaties (don’t try this with someone who can’t swim!) would reverse the process and cause the person to sink.