Oil and Water
There’s something very important about oil and water that you probably already know: oil doesn’t mix with water! That explains why oil spills on the ocean float on […]

With this science magic trick, you’ll put a new spin on our famous Seven-Layer Density Column demonstration. First, you’ll discover how to stack seven or nine layers of liquids on top of each other. That alone looks really cool, but then you take it up a notch by making different solid objects “float” in the middle of all those cool looking stacked liquids. You’ll surprise yourself and your friends with what you can do with the 9-Layer Density Tower.

Pour equal amounts of each liquid in the cups. All the amounts must be the same. You may want to set the cups in the order you’ll add them into the container: honey, corn syrup, maple syrup, whole milk, dish soap, water, vegetable oil, rubbing alcohol, and lamp oil. Add food coloring to the water and the rubbing alcohol for contrast so they stand out in the finished column.

Start your column by pouring the honey into the cylinder. It’s very important to slowly layer the honey, corn syrup, and maple syrup into the center of the cylinder. Take your time. Also, make sure they don’t touch the sides of the cylinder as you pour. It’s okay if the liquids mix a little as you are pouring. The layers will slowly even themselves out because of their varying densities.

Use the turkey baster to carefully layer the milk and the dish soap. Again, don’t let the liquids touch the sides of the container and add them s-l-o-w-l-y and carefully, a dribble at a time. Squirting them into the container is not an option if you want to end up seeing all nine layers.
Starting with the water, hold the tip of the baster against the side of the container close to the layer of dish soap. Squeeze gently so the water flows down the side of the container and onto the dish soap. Move the baster upward as needed. Layer the vegetable oil, the rubbing alcohol, and the lamp oil in the same way. Allow the layers to settle and separate even more before moving on to the next step.

Release the objects one at a time into the tower so they “slide” as gently as possible through the liquids and fall along the side of the container. Release them at different spots around the container to avoid causing too much turbulence in the same location in the liquids. This helps put the objects toward the outside of the container so you can see them on “their” layer. Start with the bolt and then release the popcorn kernel, the game dice, the cherry tomato, the plastic beads, and the soda bottle cap. Let the upper layers settle completely between each release. The cherry tomato may stir things up a bit so let it all settle down again. You may have to nudge the bottle cap a little with a straw or spoon handle so it fills with liquid and sinks. The Ping-Pong ball will float very nicely on top of the lamp oil. Well done!
Density is basically how much “stuff” is packed into a particular volume. It’s a comparison between an object’s mass and its volume. Remember the all-important equation: density = mass ÷ volume. Based on this equation, if the weight (or mass) of something increases but the volume stays the same, then density goes up. Likewise, if the mass decreases but the volume stays the same, then density goes down. Lighter liquids (like water or vegetable oil) are less dense than heavier liquids (like honey or corn syrup) so they float on top of the heavier liquids. The same amount of two different liquids you used in the container will have different densities because they have different masses. The liquids that weigh more (a higher density) will sink below the liquids that weigh less (a lower density).
To test this, you might want to set up a sensitive kitchen scale and weigh each volume of the liquids that you added to the column. Make sure that you weigh identical volumes of each liquid. You should find that the weights of the liquids correspond to each different layer of liquid. For example, the honey will weigh more than the dish soap. By weighing the same volume (e.g. ¼ cup, 60 ml) of these liquids, you will find that density and weight are closely related.
By convention, plain water has a density of 1.0 and that’s measured in g/cm3 or g/ml. So, a cubic centimeter of water has a mass of 1 gram and fills 1 milliliter of volume. If a substance has a density less than 1.0 g/cm3, it floats on water and one with a density more than 1.0 g/cm3, it sinks in water. The numbers below are based on data from manufacturers for each item. Since each manufacturer has its own “secret” formula, densities may vary slightly from brand to brand for the same product but these are pretty close.
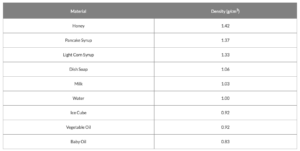
The same rule for “sink or float” goes for the small objects that you dropped into your density column. The metal bolt is more dense than all of the liquids in the column and therefore sinks directly to the bottom. Less dense objects will float on individual layers up the column. For instance, the plastic beads are more dense than the vegetable oil and everything above it but less dense than the water and everything below it. This makes the beads settle on top of the water. The Ping-Pong ball is the least dense of it all and floats on top.
This is the perfect opportunity to get in some scientific exploration. Use what you learned from dropping the beads, soda bottle cap, tomato, and game die into the container to figure out which items are more or less dense than water. Which items have more density than vegetable oil? What items are less dense than honey? You could make a hypothesis about the density of an object and then test it in the column. How do the data support or not support the results?
When all the liquids and small objects have been added to your density tower, you will have what appears to be a “magic” column but it’s all based on science. All of the liquids will be clearly distinguishable from each other and each of the objects will have settled at different levels within the column. Construct a table to show the order that the liquids and objects are in at the start. Be sure to record the date, too. If you leave it undisturbed over time, some interesting changes may occur in the column. Keep track of what you see with notes and photos with dates. Science fair anyone?