Fireproof Balloon
Common sense tells you that it’s impossible to boil water in a paper bag, but this classic parlor trick was a favorite of Victorian magicians. […]

When is the last time you made bubbles catch on fire? Probably never . . . but then again, this is the teachers-only section of our labs, so anything is possible. Fire Bubbles is the signature activity for my book, Fire Bubbles & Exploding Toothpaste (because it appears on the cover!), but it’s also one of my personal favorites and one that I perform on television all the time. I honestly never thought that talk show host Ellen DeGeneres would allow me to light her hands on fire, but she did . . . and the audience went wild! Aside from the gee-whiz factor of Fire Bubbles, this demonstration brilliantly illustrates the heat-conducting properties of water and shows how even a very thin layer of water protects your skin from the blistering flames.
WARNING! TEACHERS ONLY! This demonstration is provided for educational purposes only and should not be attempted if you are not a properly trained professional. If you choose to perform this demonstration, safety glasses, a fire extinguisher, and a friend to help you are all required.
NOTE: This demonstration requires that you have an available source of methane gas. That’s why it’s best suited for chemistry teachers who have easy access to gas jets in the laboratory.

You’ll want to take the beach ball with you to the hardware store as you look for the right size of rubber tubing (you’ll find it in the plumbing section). Your mission is to select tubing that will tightly fit into the opening of the beach ball. It should be a very snug fit to keep the gas from leaking out. A helpful tip is to put a few drops of dish soap on the end of the tubing to act as a lubricant as you push the end of the tubing into the beach ball. Just feed the tube in a few inches in order to secure it in place.

It’s best to start by finding a clear plastic jar similar to the one pictured in the photos. Start by filling the plastic jar about one-half full with ordinary tap water. Add a squirt of dish soap to the water.
It’s important to make sure that the beach ball is empty (all of the air is squeezed out) before filling it with methane gas. Again, you’ll need to have access to a natural gas jet (the gas is methane) in the school chemistry lab. Hold the tube up to the jet and fill the beach ball with methane gas. Place your finger over the opening of the tube (or use a bulldog clip to crimp the hose) to keep the gas from escaping while you transport it over to the jar of soapy water.

Submerge the open end of the tube in the soapy water. Secure the tube at the opening of the jar with a piece of duct tape, or you can simply drill a hole in the plastic jar beforehand and feed the tubing through the opening as a way to keep it in place. The latter method is shown in the accompanying photos. Don’t worry about a small amount of the gas coming out of the beach ball when you submerge the tubing. It’s normal for this to happen.

It’s time for your friend to step up and help out. Both of you need to put on your safety glasses. Your helper is in charge of squeezing the beach ball in order to generate bubbles of methane gas in the plastic jar. Squeeze the ball with gentle but constant pressure to generate some bubbles.

Fill the large bowl or plastic storage container one-half full with plain water.
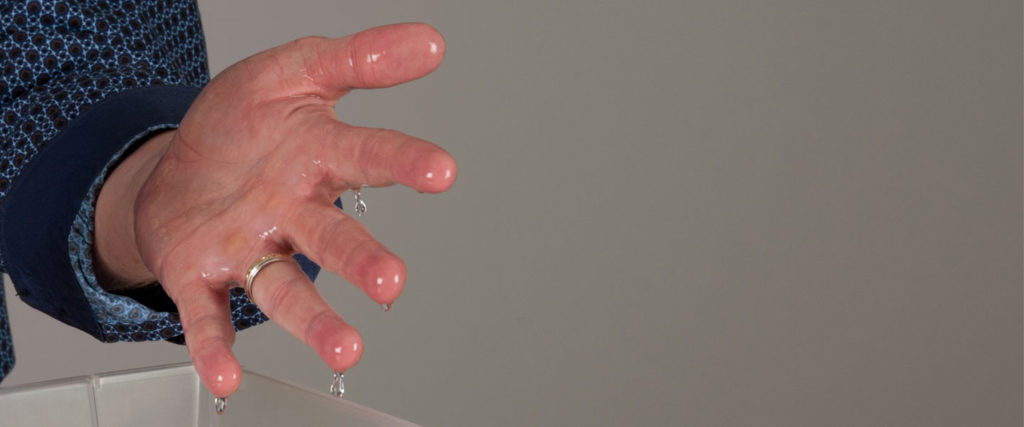
While your friend is squeezing the beach ball, submerge both of your hands in the container of plain water. The goal is to make sure every part of your hands and wrists are covered with water. Failure to get your hands and wrists completely wet can result in a burn . . . so make sure to douse them with water.

By this time, the bubbles of methane gas should be rising out of the plastic jar. Scoop up a small handful of bubbles with your left hand. Step away from your friend. Light the striker with your right hand and touch the bubbles in your left. (If you choose to scoop up bubbles in both hands, then your beach ball friend will have to be the one who lights the fire.) This is probably the scariest step in this entire book of demonstrations! The methane-filled bubbles will produce a large flame (keep your hand in front of you and don’t move!), but it will extinguish on its own in just a few seconds, and your hands will remain unharmed! Scream with excitement and take a well-deserved bow.

Within seconds, you’ll hear the chant, “Do it again, do it again!” Give your fans what they’re asking for and do it again, but first make them offer up their best hypothesis as to why you didn’t get burned. And make sure you douse your hands in the container of plain water again before your encore performance.
If you’ve attempted the Fireproof Balloon activity, you already have a good idea as to how this works. If not, it’s a great idea to present the Fireproof Balloon activity prior to performing Fire Bubbles to see if any of this science is actually sinking into the brains of your audience members. Water is a great conductor of heat, and it literally pulls the heat away from your hand for the few seconds that the methane bubbles are on fire. As the flame heats the water on your hands, some of the water at the very surface evaporates, and it’s this natural cooling process from the evaporation that keeps your hand from burning.
WARNING! TEACHERS ONLY! This demonstration is provided for educational purposes only and should not be attempted if you are not a properly trained professional. If you choose to perform this demonstration, safety glasses, a fire extinguisher, and a friend to help you are all required.
It must be said that if you play with fire, you’ll eventually get burned. I wish that I could call it Spangler’s Law of Doing Science Demonstrations That Use Fire, but I think that it’s probably attributed to someone else. Nevertheless, you must use extreme caution when doing this activity. It’s only natural to grow more and more confident as you repeat the demonstration, and you’re likely to want to set fire to a huge column of bubbles on your hand. Don’t do it! If the heat from the flame is greater than the water’s ability to pull the heat energy away from your hand, you’ll get burned (literally and figuratively).
When presented in the appropriate learning environment, using all of the necessary safety equipment (and common sense), this demonstration is guaranteed to become one of your all-time favorites.