Bouncing Bubble
With the Bouncing Bubble, that little puff of air trapped in a thin soap film will bounce. What?!?! It’s true! These bubbles are formulated to […]
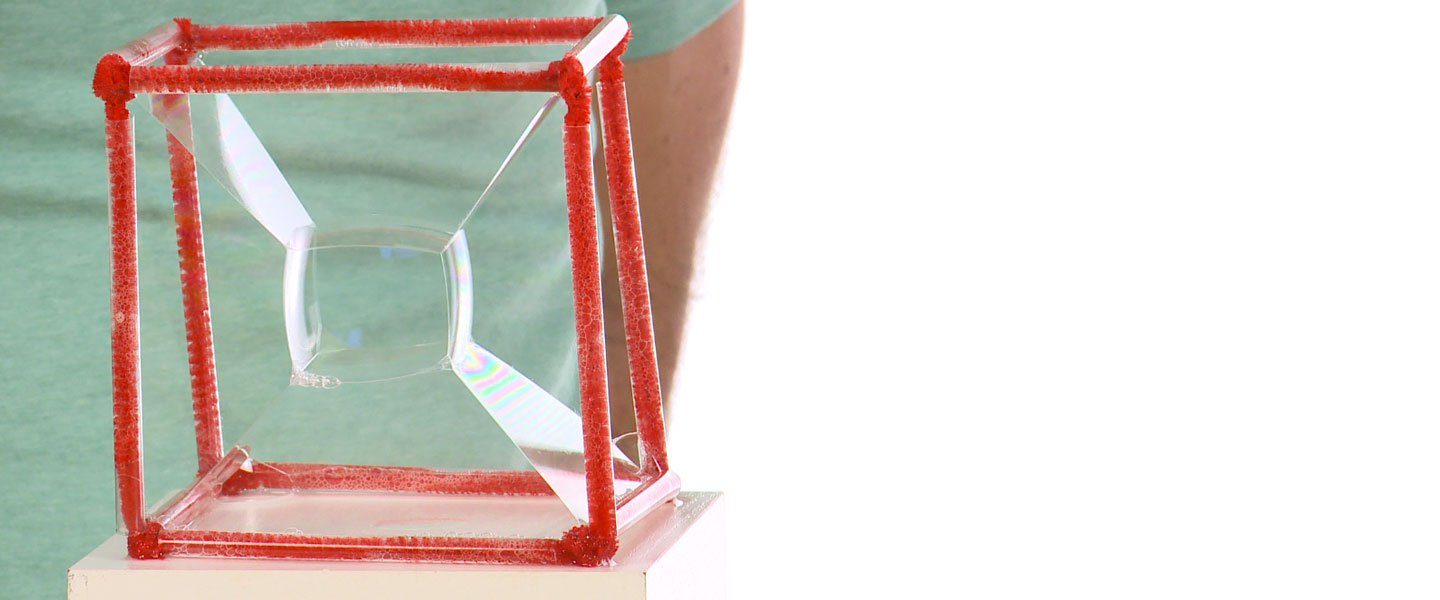
Soap bubbles that are not spherical are unusual and they don’t happen without a little help. Nature is funny like that. Square bubbles are easy to make and can serve as a great learning tool to explore solutions, soap films, and surface tension. You build a bubble maker that’s a cube, dip it into some soap solution, and the film stretches out flat. The film clings to the edges of the cube causing the bubble to appear to be a square. Well, it’s almost a square.
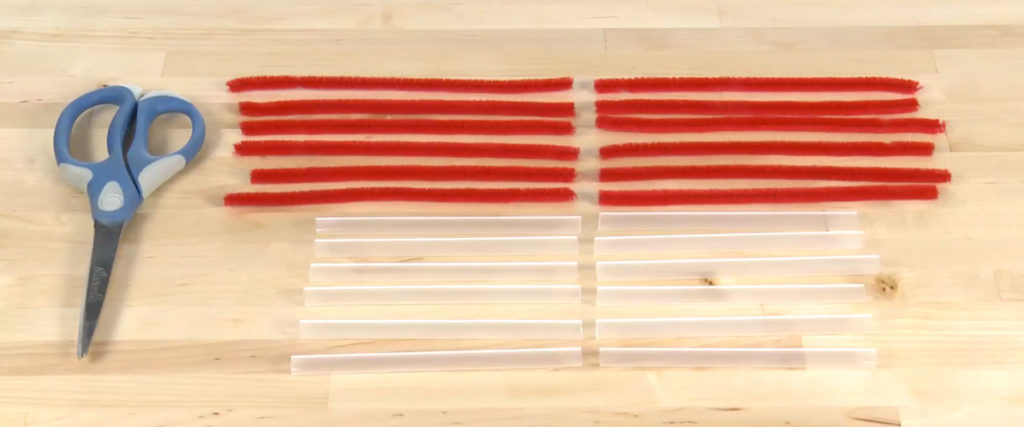
Use the scissors to cut each of the six pipe cleaners and straws in half. You’ll have twelve pieces of each.
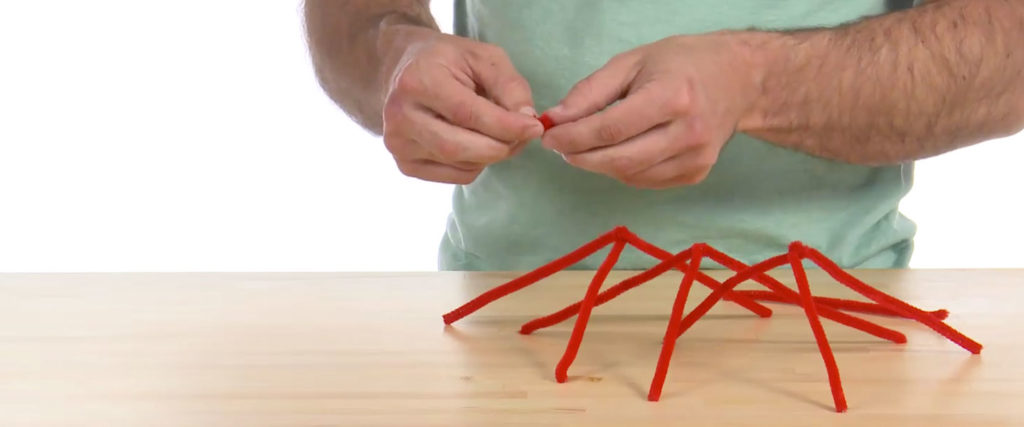
Twist three of the fuzzy sticks together at an end to make a triangular, pyramid-shaped component. You’ll end up with four, three-legged pieces

Slide a piece of straw onto each fuzzy wire leg. Some of the fuzzy wire should stick out of the straw on each leg.
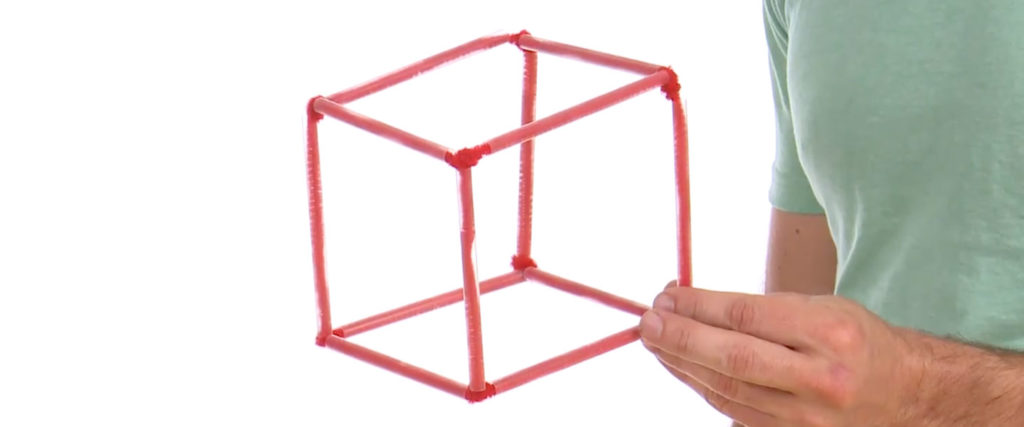
Build the cube by twisting the fuzzy wire ends on one component to the ends on another component. Connect the legs until the cube is complete. Make it as even a shape as you can.

Mix the bubble solution. Check out the “Making Bubble Solution” suggestions below.

When you and your bubble solution are ready, dip the cube shape into the solution. Let it sit in the solution a few seconds and lift it out by holding on to two corners. Giving the cube a gentle shake helps the soap film even out itself and causes excess solution to drip back into the bucket. Set the “square” bubble on a flat surface to keep the bubble film stable.
You can gently shake the cube to change which way the film faces or just rotate the cube to make sure the square bubble in the center is horizontal. You need to see the square when you look down into the cube from above it.
Use a bubble wand you have or make the pipette into a wand by cutting off half of the bulb end. Blow a bubble and drop it into the center of the square. Voilá! The bubble you dropped into the cube “magically” transforms from a sphere to a bulging cube. Now that’s a square bubble!
Bubbles form because water has reduced surface tension in the presence of soap. Hydrogen atoms in one water molecule are attracted to oxygen atoms in another water molecule (cohesion). They like each and they cling together. Soap molecules help them be more “stretchy” or flexible by butting in and decreasing the force of the attraction. Soap (and the glycerin) also reduces evaporation of water molecules so individual bubbles can last longer. There is also some strengthening of the soap film.
Why are bubbles always round? Physicists will tell you that bubbles use a minimum amount of surface area needed to enclose the volume of air trapped inside. In this activity, however, as you dip the cube into the bubble solution, the solution stretches between the edges and the soap film clings to the sides of the cube (adhesion). This causes the bubbles to appear square or cubic. The soap film connects the shortest possible distance while still connecting all sides. Notice, however, that even the bubble you blew into the center at the end bulges slightly on its sides. Bubbles love a spherical shape!
Making Bubble Solution
Use a container that holds about 2 gallons (7.5 L) of water.
*Perfectly good bubbles can be made without adding glycerin.