Ice Tray Battery
You probably know that voltaic batteries come in all kinds of shapes and sizes, from tiny button batteries to car batteries to huge industrial heavy-weights. […]
Voltaic batteries of all shapes and sizes are devices that convert chemical energy into electrical energy. You probably use batteries to power your cell phone, iPod, or any number of other gadgets. But, you can actually use chemical energy stored in a lemon and two metals to make a current and light up a small LED. It’s true and we’ll show you exactly how it’s done.

Use a kitchen knife to cut a penny-sized slit in all four lemons.

Insert a penny halfway into each of the four slits you cut.

Push a zinc-galvanized nail into each of the lemons, opposite the penny. Don’t let the nail and penny touch in or out of the lemon.

Use a set of alligator clips to connect a nail on one lemon to a penny on another lemon.
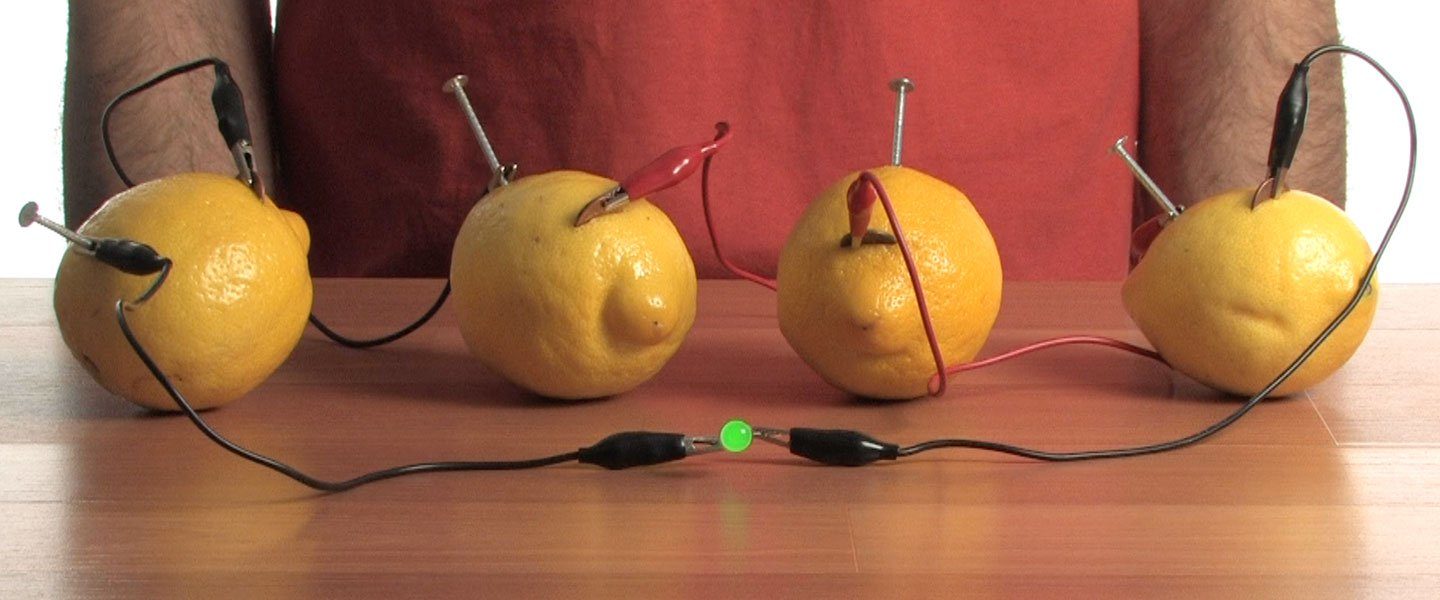
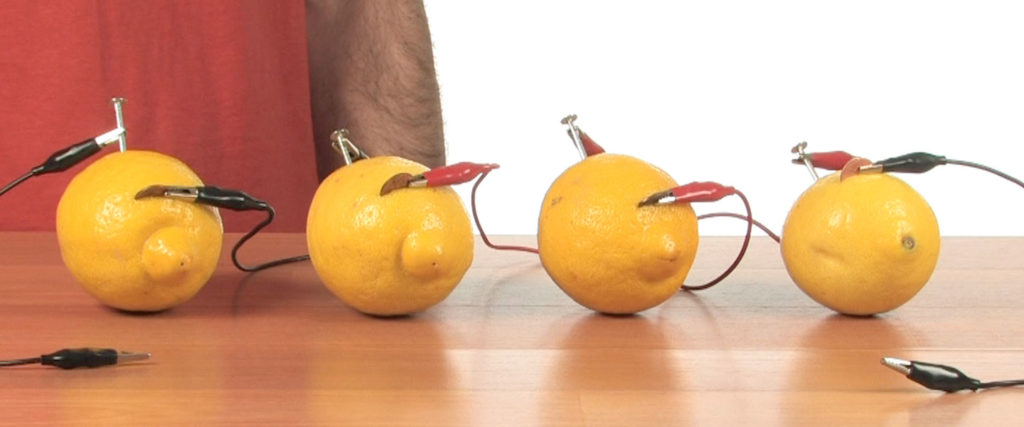
Connect all four lemons together with alligator clips. Each set of alligator clips should connect a nail to a penny.
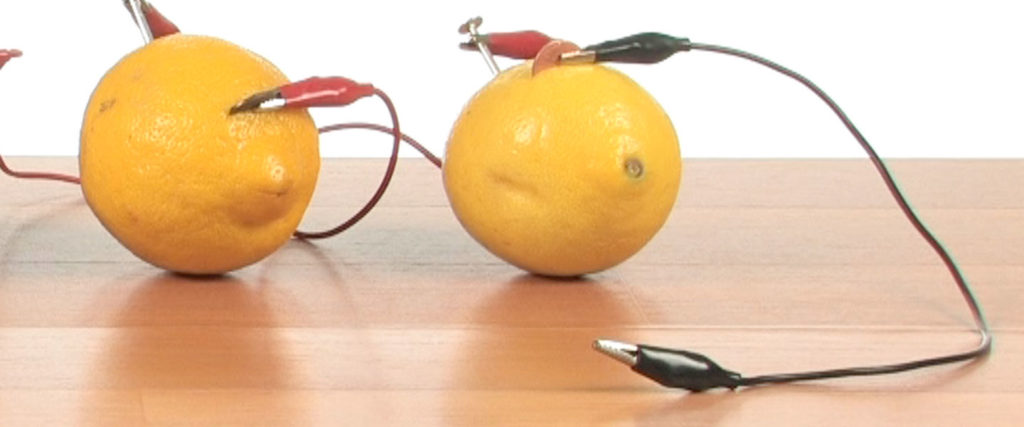
Connect one of the last alligator clips to the only open penny.
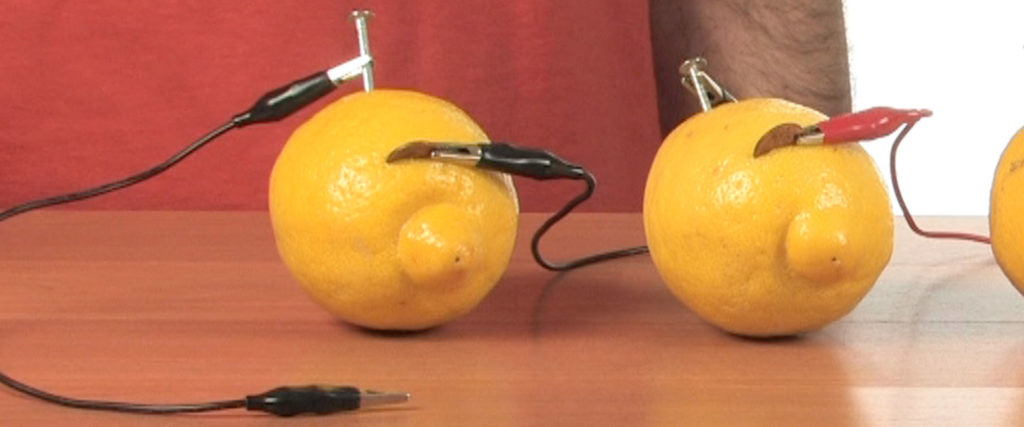
Connect the other clip to the only open – duh! – nail. Now you have two unused clips on two separate alligator clips. You know what to do!
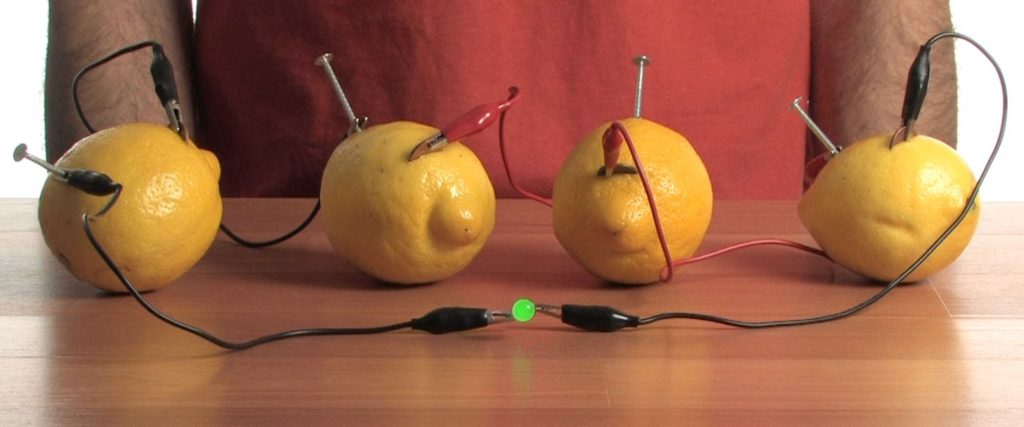
Attach the two loose alligator clips to the LED. Check it out! The chemical energy in the lemons has been used to power the LED with electrical energy.
Batteries are comprised of two different metals suspended in an acidic solution. With the Fruit-Power Battery, the two metals are zinc and copper. The zinc is in the galvanization on the nails, and the pennies are actually copper-plated zinc. The acid comes from the citric acid inside each lemon.
The two metal components are electrodes, the parts of a battery where electrical current enters and leaves the battery. With a zinc and copper setup, the electron flow is out of the penny (copper) and into the nail (zinc) through the acidic juice inside the lemon. In the exchange of electrons between the zinc and the copper over the acid bridge, copper accepts two electrons from zinc which accounts for the current.
Once the Fruit-Power Battery is connected to the LED, you’ve completed a circuit. As the electrical current passes through the LED, it powers the LED and then passes back through all of the lemons before getting to the LED again. By the way, an LED is polar sensitive. That means an LED will glow only if the current is flowing through it in the right direction. If you hook up the LED and it doesn’t glow, switch the alligator clips attached to its legs. That should do it.