How to Make Slime - Elmer's Glue Recipes
Expert slime makers agree that Elmer’s glue makes the best DIY slime. However, there are some tricky variations that can turn your slime from just […]

If you’re old enough to remember Silly Putty, you may remember what it felt like to roll it up into a ball in your hand. The fascinating thing about Silly Putty is that you can put a ball of it down on the table and, eventually, it spreads itself out. How does it do that? Here’s a simple way to make your own version of this popular toy called GAK using household materials. You’ll use the recipe to make a white and a blue batch of GAK, give them a little twist, and use the concoction to learn more about the movements of real glaciers.

You’ll make two separate batches of GAK—one will be white and the other will be blue. You could purchase one large container of Elmer’s Glue-All and follow the recipe or simply use two 8-ounce bottles. The measurements do not have to be exact, but it’s a good idea to start with the suggestions below for the first batch.

This recipe is based on using a brand-new, 8-ounce (240 mL) bottle of Elmer’s Glue-All. Empty the entire bottle of glue into a mixing bowl. Fill the empty bottle half full with warm water and shake (okay, put the lid on first and then shake). Pour the glue-water mixture into the mixing bowl and use the spoon to mix well.

Measure 1/2 cup (120 mL) of warm water into the plastic cup and add a heaping teaspoon of Borax powder to the water. Stir the solution. Don’t worry if all of the powder dissolves.

While stirring the glue in the mixing bowl, slowly add a little of the Borax solution you just made. Immediately you’ll feel the long strands of molecules start to connect. It’s time to abandon the spoon and use your hands to do the serious mixing. Keep adding the Borax solution to the glue mixture (don’t stop mixing) until the GAK has a putty-like consistency. You should be able to roll it on the table like dough, but if you let it rest for a couple of minutes, the GAK will spread itself out. Set this batch of white GAK off to the side while you mix up the next batch.

Repeat the four preceding steps to make your second batch of GAK, but this time add about ten drops of blue food coloring during Step 2. As you’re adding the Borax solution, try to keep the consistency of this batch the same as that of the one you previously made.
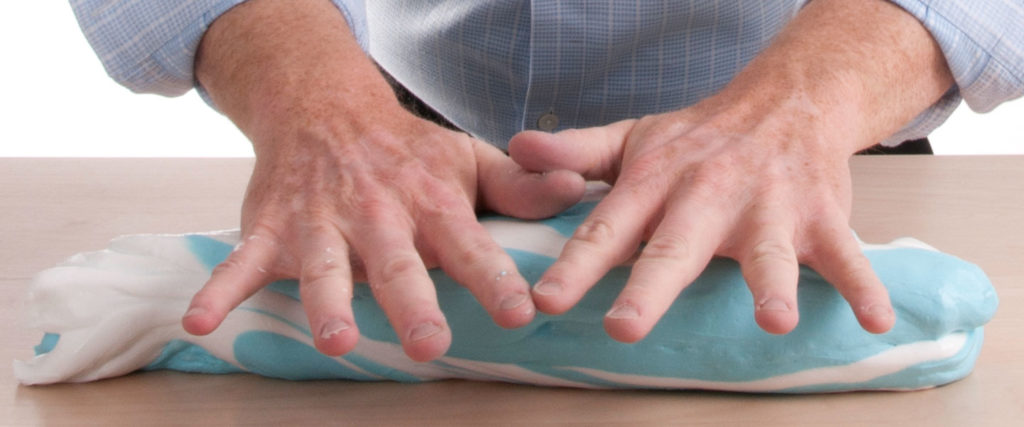
Here comes the fun part of combining the blue and white batches of GAK. There’s no right or wrong way to do this—just twist and fold the large pieces until you get a cool swirl of blue and white GAK.

Lay the GAK out on a cookie sheet or plastic tray. Use a few books to prop up one end of the cookie sheet. This will allow the GAK to begin to flow slowly downhill. The mixture of blue and white GAK creates some amazing patterns as the mixture flows downward, simulating the slow movement of a glacier.

At any point you can pick up the GAK, reshape it into a big blob, set it at the top of the cookie sheet, and restart the flow. Try placing various sizes of rocks on the cookie sheet as obstacles for the GAK to flow around. Depending on the size of the rock, the movement of the Glacier GAK might be powerful enough to push the rock down the hill. You can read more about the science of glaciers in the “Real-World Application” section.

When you’re finished playing with your GAK, seal it up in a zipper-lock bag for safekeeping.
The mixture of Elmer’s Glue-All with Borax and water produces a putty-like material called a polymer. In simplest terms, a polymer is a long chain of molecules. You can use the example of cooking spaghetti to better under- stand why the GAK polymer behaves the way it does. When a pile of freshly cooked spaghetti comes out of the hot water and into the bowl, the strands flow like a liquid from the pan to the bowl. This is because the spaghetti strands are slippery and slide over one another. When the water drains off of the pasta, the strands start to stick together and the spaghetti takes on a rubbery texture. Wait a little while longer for all of the water to evaporate, and the pile of spaghetti turns into a solid mass. Drop it on the floor and watch it bounce.
Many natural and synthetic polymers behave in a similar manner. Polymers are made out of long strands of molecules like spaghetti. If the long molecules slide past each other easily, then the substance acts like a liquid because the molecules flow. If the molecules stick together at a few places along the strand, then the substance behaves like a rubbery solid called an elastomer. Borax is the compound that is responsible for hooking the glue’s molecules together to form the putty-like material. There are several different methods for making this putty-like material. Some recipes call for liquid starch instead of Borax soap. Either way, when you make this homemade Silly Putty you are learning about some of the properties of polymers
Adjust the amounts of water, Borax, and glue to see how the consistency of the GAK changes. Remember to change only one ingredient at a time to best determine the effect each ingredient has on the overall consistency of the putty.
For hundreds of thousands of years, the movement of glaciers has shaped land through erosion and deposition, creating landforms such as U-shaped valleys, moraines, and kettle lakes, to name a few. Motion and change define a glacier’s life, and glaciers grow and shrink in response to a changing climate. Currently, glacial retreat is implicated in the Earth’s changing climate patterns and may have a great impact on sea levels and weather cycles.
The unique, slow moving properties of the GAK simulate the movement of a glacier. At a molecular level, ice is comprised of stacked layers of molecules with relatively weak bonds between the layers. This is similar to the makeup of our GAK molecules. Ice can stretch or break depending on the amount of pressure applied. If there is a lot of pressure or a high strain rate, ice will crack or break (causing crevasses in glaciers). When the pressure is lower or the strain rate is small and constant, ice can bend or stretch. The steady pressure from the bulk of the ice mass and the pull of gravity cause the glacier to flow slowly (so slowly you can’t see it) downhill, bending like a river of ice.
Scientists study glaciers because by their movement, glaciers mark change over an incredible period of time. By monitoring glaciers around the world over time, researchers construct valuable records of glacial activity and their response to climate variation.