Disappearing Ink - FriXion Pen
Give someone special a note that requires heat to decode the message. You won’t believe what the ink in this pen will do. Your message […]
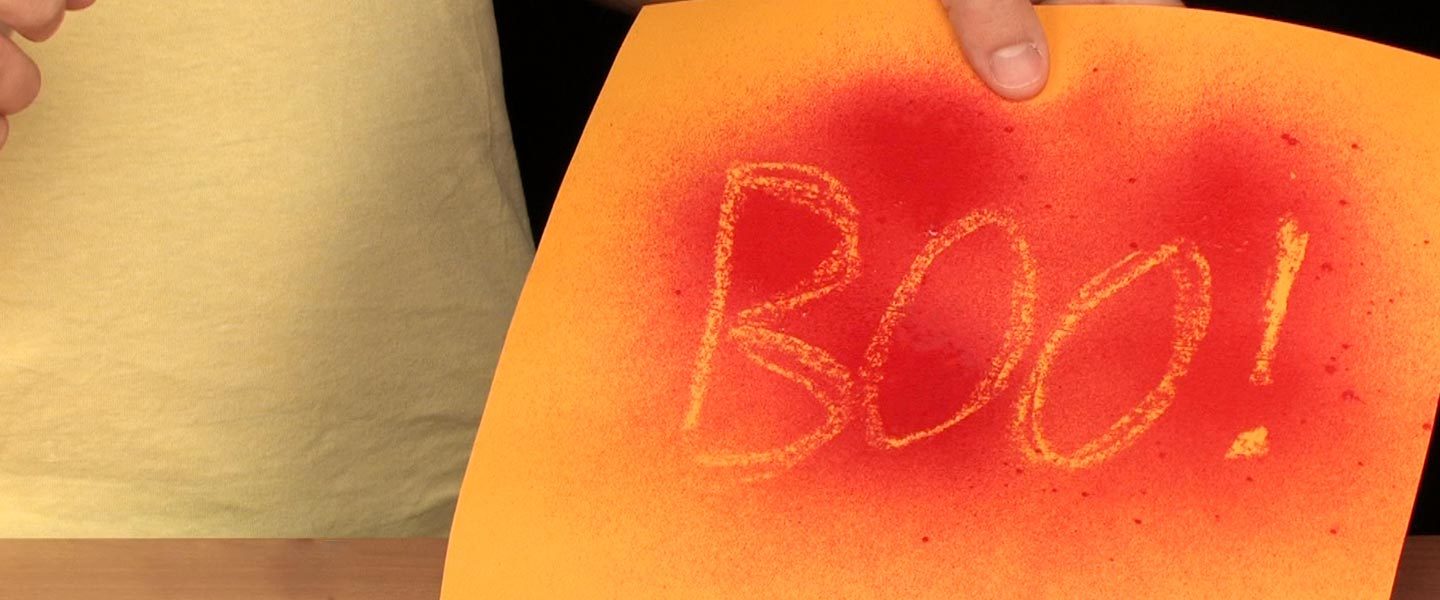
The term goldenrod is typically used to describe a color of paper – golden yellow. However, some brands of goldenrod paper contain a special dye that turns bright red when exposed to solutions that are basic, like ammonia, water, or washing soda. Learn how to use this special color-changing paper to share a hidden message or to create a creepy bleeding paper experience.
Place a piece of Goldenrod Paper on the table. Make sure that the table is clean and the work surface is dry.

Use the old piece of wax candle to write a secret message (such as “Hi!” or “WOW”) on the bottom half of the paper.

Fill a jar with a small amount of ammonia water. Dip a cotton ball in the ammonia water and wipe it across the bottom portion of the Goldenrod Paper.

As you continue to wipe designs on the Goldenrod Paper, notice that the paper does not stay red forever.
Place a piece of Goldenrod Paper on a clean, dry surface.
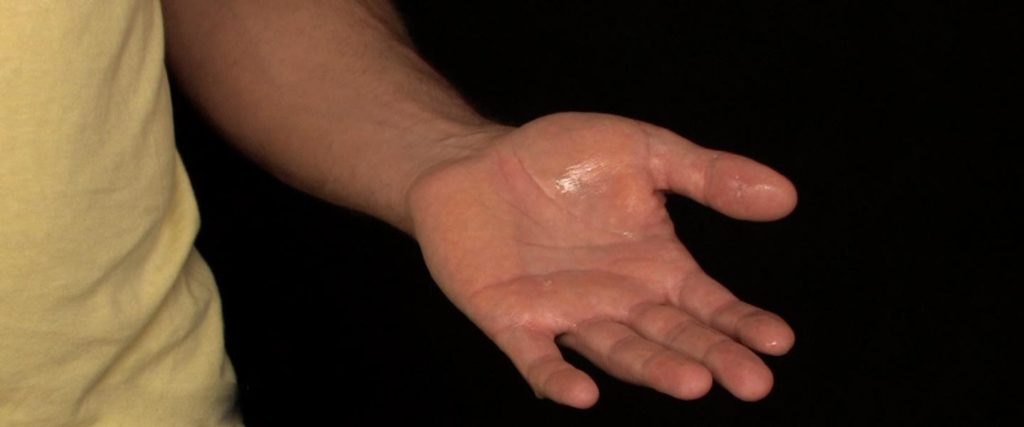
Away from the paper, use the cotton ball to wet your hand with the ammonia solution. Lab Safety: Remember to never put lab chemicals near your eyes, ears, nose, or mouth and always ask an adult helper to assist you in your science explorations.

Gently slap your hand down on the Goldenrod Paper… oh no! It’s a bleeding handprint!

Notice once again that the paper does not stay red forever. Oh, be sure to wash your hands, too.
The ammonia on the cotton ball is a base and causes the dye in the special Goldenrod Paper to change color. You probably noticed that the red color fades over time and the paper eventually changes back to its original yellow color. Why? Some of the carbon dioxide gas (CO2) in the air we breathe mixes with water vapor and makes a weak acid (carbonic acid). It’s slightly on the acidic side of the pH scale. This reacts with the ammonia on the paper to produce ammonium carbonate. That gradually changes the pH of the indicator to neutral (roughly a pH of 7) and the dye changes back to yellow.
If you use a stronger base like washing soda (also known as sodium carbonate, which can be found on the detergent aisle at your local grocery store), the red message will not disappear with just the carbon dioxide in the air. You will need to use a stronger acid like lemon juice or vinegar to change it from red back to yellow.
Now that you have started your exploration of acids and bases, you can keep investigating with safe household products you will find at home. Try using this special golden rod paper as pH indicator paper to classify your household products as being either acidic or basic. Simply use a cotton ball and test the solutions on different sections of a new sheet of goldenrod paper. Did the paper run red? Did the paper turn back to yellow? What conclusions can you draw about these household products from your testing?
Household ammonia is a pretty good cleaner because it’s a strong base. You have to be careful with it, however, and use small, diluted quantities of it. Dilute it one-to-one with water to make your test solution above. Ammonia really stinks and its fumes can damage your sniffer with too much exposer. It makes sense, then, that you need to be in a well ventilated area when you use it. Also, never mix ammonia with other household cleaners. Dangerous gases can be released in the reaction and your pH testing career will come to an unexpected halt. When in doubt, ask an adult science helper!