Red Cabbage Chemistry
Ahh, the sweet smell of science! This next activity is super smelly, but really cool activity. Plug your nose and get ready to make your […]

You no longer need to search for specialty litmus paper. Steve and his team have come up with a formula that will allow you to create your own acid and base detecting litmus paper using little more than a bit of fruit.

Remove any stems or leaves from your berries and place them in the bag.
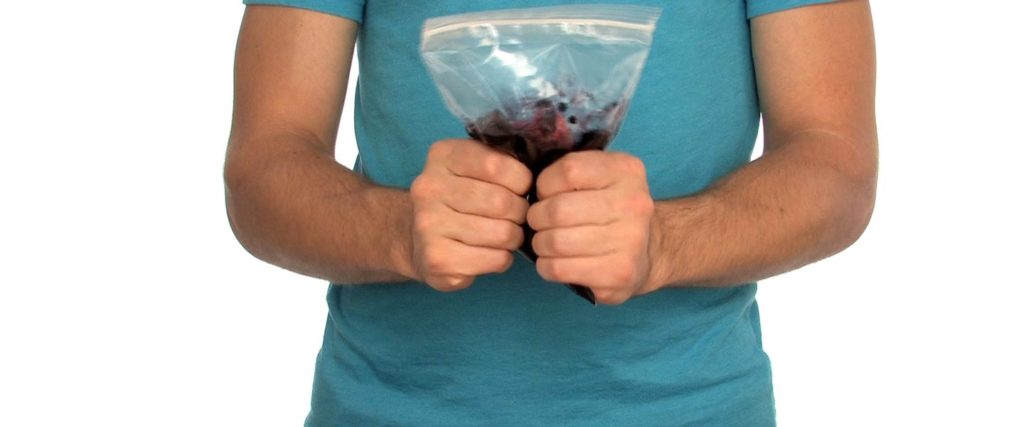
Zip up the bag and mash and mush your berries until they look like jam.

Add a small amount of water to thin the juice.

Mix it all up and pour your berry liquid into a bowl.

Cut some thin strips of the white construction paper and dip them into your mashed berries. Push the strips all the way into the berry mush to make sure they are good and coated with the juice.

After taking your well-soaked paper out of the juice, pull the strips between your thumb and index finger to remove any excess juice and pulp.
Lay the strips onto paper towels and allow them to dry.

Once your paper strips have dried, carefully pick off any large pieces of pulp or berry skins. You’re ready to use your Berry pH Paper.
To test your Berry pH Paper, mix 1/4 cup water and 2 TBSP of dish soap in a clean cup.
In a separate cup, pour 1/4 cup of vinegar.

Dip half of one strip of your Berry pH Paper into the bowl containing dish soap and water.
Do the same thing with another strip of Berry pH Paper, but this time dip it into the vinegar.

Set the Berry pH Paper strips onto a piece of paper towel to dry. This should only take about five minutes. Make sure you label the pH strips to remember which strip was dipped in which liquid.
Blackberries, blueberries, strawberries, and a bunch of other flowers, leaves, and stems are naturally occurring pH indicators. This is true because they contain chemicals from the anthocyanin family of compounds. Anthocyanin compounds turn red in acids and blue in bases when they are in their pure form. In this case, we have the anthocyanin compounds within the juice of the berries. This results in a less distinct, but still distinguishable, color change. Blackberry pH Paper turns pinkish red in acids and turns deep purple in bases.
Creating Berry pH paper is pretty cool, but it isn’t a science fair project. You can create a science fair project by identifying a variable, or something that changes, in this experiment. Let’s take a look at some of the variable options that might work:
That’s just one idea, but you aren’t limited to that! Try coming up with different ideas of variables and give them a try. Remember, you can only change one thing at a time. If you are testing different liquids, make sure that the other factors are remaining the same!