Eye Dropper Cartesian Diver
Is it mind control or just a clever science trick? It’s a classic science experiment using an eye-dropper, a soda bottle filled with water, and […]
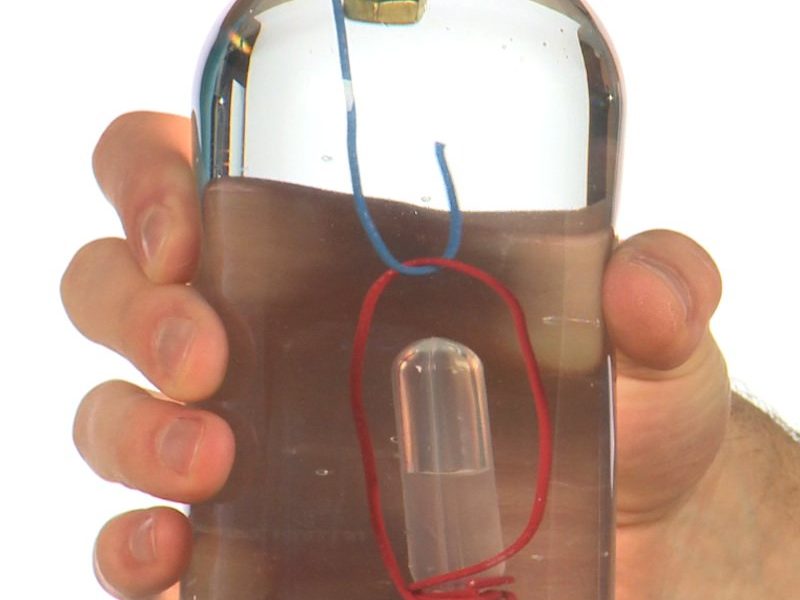
Hook is an amazing game that combines cool science with coordination and skill. You’ll make a special diver, float it in a soda bottle tank and then use your talent to retrieve sunken treasure! Hook is an updated version of a classic science experiment called a Cartesian Diver. Get ready to challenge your friends to a game of Hook! Discover the relationship between air pressure and density, and how to make a floating diver more or less buoyant. Float or sink? It’s your choice!
Make another Cartesian Diver, with the directions in the first activity. (You need two divers to play Hook.)
The Cartesian Diver is a classic science experiment that’s hundreds of years old. It’s named for a Frenchman, René Descartes (1596-1650), who made huge contributions in the fields of philosophy, math, and science. The original Cartesian Divers were made out of glass medicine droppers or delicate glass ampules.
When you have the water levels adjusted correctly in your new, unbreakable divers, you should see the water in the diver rise as you squeeze the bottle. The air trapped in the pipette compresses into a smaller space and the diver’s weight increases. It becomes less buoyant and it sinks. When you release the squeeze, the compressed air expands and forces water out of the diver, allowing it to float to the top of the bottle.
Note: If the bottle requires a super-strong squeeze to move the diver, there isn’t enough water in the pipette. Remove the diver from the bottle and increase the water level in the diver so it just barely floats.