Color Mixing Gobstoppers (Candy Science)
Hard candies are known for their bright colors, delicious tastes, as well as having a ton of sugar. While munching on a handful of candy […]

This unforgettable hands-on learning experience is fun and easy—no wonder it’s one of Steve Spangler’s most popular experiments! Learn how to turn this activity into an awesome science fair project.

Pour enough milk in the dinner plate to completely cover the bottom to the depth of about 1/4 inch. Allow the milk to settle before moving on to the next step.

Add one drop of each of the four colors of food coloring—red, yellow, green, and blue—to the milk. Keep the drops close together in the center of the plate of milk.

Find a clean cotton swab for the next part of the experiment. Predict what will happen when you touch the tip of the cotton swab to the center of the milk. It’s important not to stir the mix—just touch it with the tip of the cotton swab. Go ahead and try it.
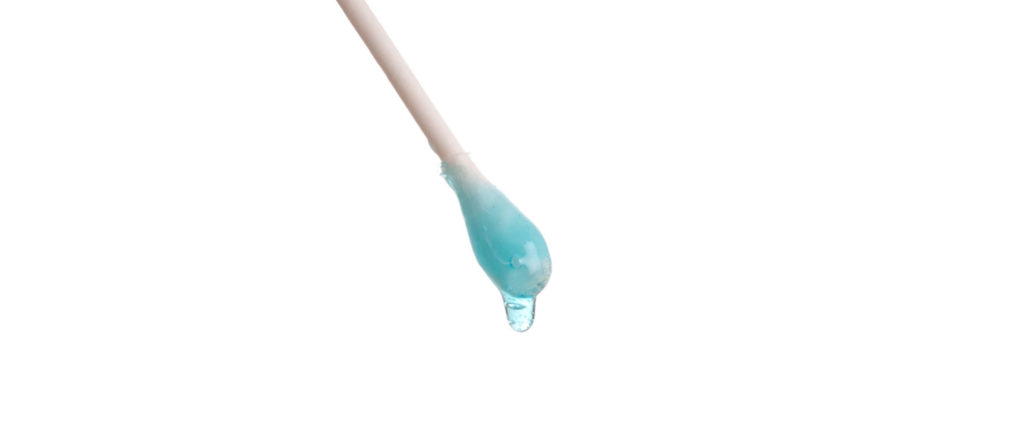
Now place a drop of liquid dish soap on the other end of the cotton swab. Place the soapy end of the cotton swab back in the middle of the milk and hold it there for 10 to 15 seconds. Look at that burst of color! It’s like the Fourth of July in a plate of milk.

Add another drop of soap to the tip of the cotton swab and try it again. Experiment with placing the cotton swab at different places in the milk. Notice that the colors in the milk continue to move even when the cotton swab is removed. What makes the food coloring in the milk move?
Milk is mostly water, but it also contains vitamins, minerals, proteins, and tiny droplets of fat suspended in solution. Fats and proteins are sensitive to changes in the surrounding solution (the milk).
The secret of the bursting colors is in the chemistry of that tiny drop of soap. Like other oils, milk fat is a non-polar molecule and that means it doesn’t dissolve in water. When soap is mixed in, however, the non-polar (hydrophobic) portion of micelles (molecular soap structures in solution) break up and collect the non-polar fat molecules.Then the polar surface of the micelle (hydrophilic) connects to a polar water molecule with the fat held inside the soap micelle. Thanks to the soap connection, literally, the non-polar fat can then be carried by the polar water. This is when the fun begins.
The molecules of fat bend, roll, twist, and contort in all directions as the soap molecules race around to join up with the fat molecules. During all of this fat molecule gymnastics, the food coloring molecules are bumped and shoved everywhere, providing an easy way to observe all the invisible activity. As the soap becomes evenly mixed with the milk, the action slows down and eventually stops. This is why milk with a higher fat content produces a better explosion of color—there’s just more fat to combine with all of those soap molecules.
Try adding another drop of soap to see if there’s any more movement. If so, you discovered there are still more fat molecules that haven’t found a partner at the big color dance. Add another drop of soap to start the process again.
Repeat the experiment using water in place of milk. Will you get the same eruption of color? What kind of milk produces the best swirling of color, skim, 1%, 2%, or whole milk? Why? This is the basis of a great science fair project as you compare the effect that the dishwashing soap has on a number of different liquids. Do you see any pattern in your observations?