Oil and Water
There’s something very important about oil and water that you probably already know: oil doesn’t mix with water! That explains why oil spills on the ocean float on […]
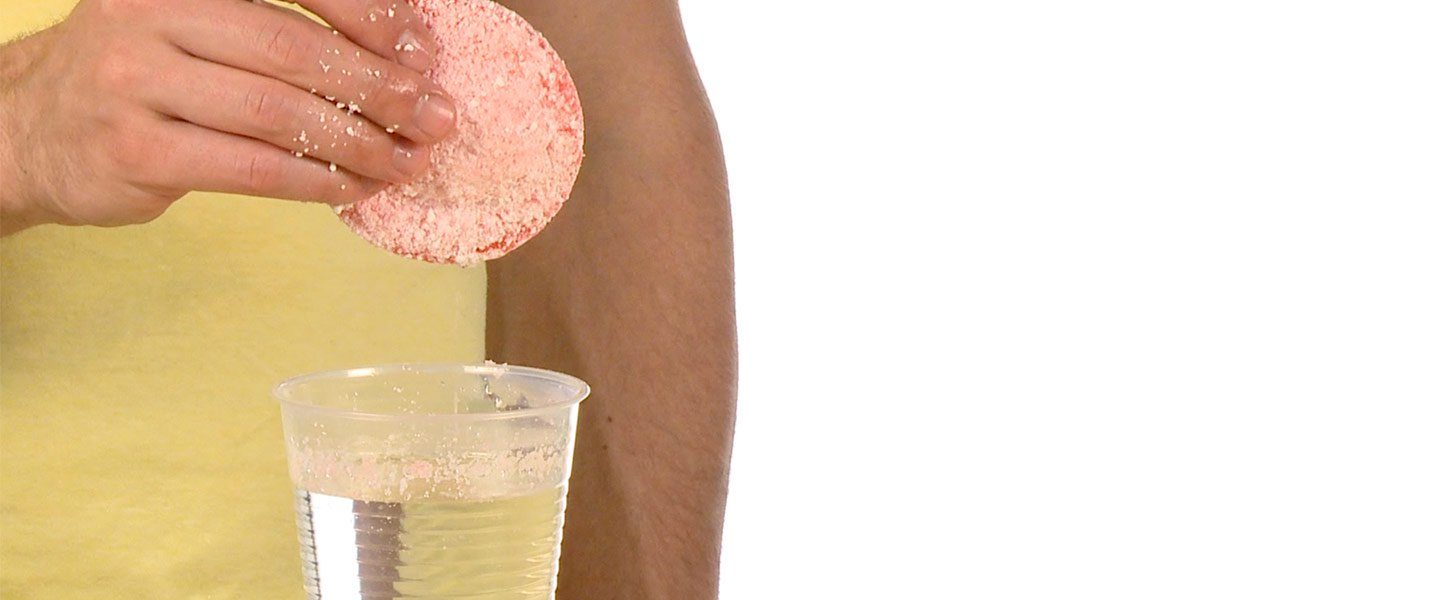
Imagine a simple way to clean up an oil spill: A small amount of a non-toxic powder is sprinkled onto the spreading oil. In seconds, the powder collects the oil and forms a sponge-like layer that can be easily skimmed off of the water. It’s more than just a dream! It’s a new way of using superabsorbent polymers to change the way cleanup teams control oil spills and manage other waste problems. The results are amazing!
Marvel Mystery Oil® is a registered trademark of Turtle Wax, Inc., Chicago, USA. All Rights Reserved.

Fill the clear cup 3/4-full with room temperature water.

Add enough Marvel Mystery Oil to the water to make a thin, but obvious, oil slick on the water. Since petroleum is less dense than water, it floats on top of the water. Its bright color reveals it clearly.
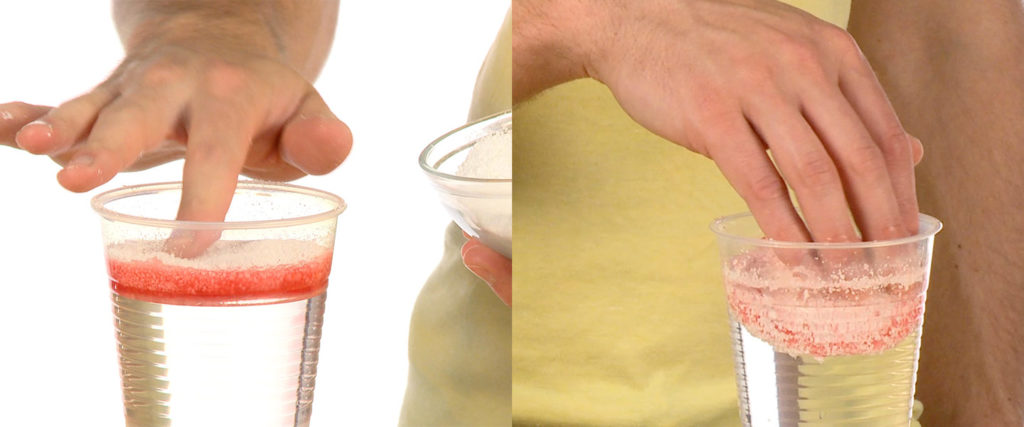
Spread the polymer evenly over the surface of the oil. The polymer immediately bonds to the oil and forms a sponge-like disk that floats on the water.
Leave the polymer in place for about 2 minutes. You’ll want to watch this process closely, too. Before removing the oil/polymer glob, you may need to press down on the edges of the polymer where it touches the cup. It may stick to the plastic in the cup.
Lift the polymer disk out of the cup and place it on a clean piece of waxed paper to “cure.” As water dries out of the disk, the polymer becomes firm and rubbery – like a sponge cake. Any remaining hydrocarbon molecules find bonding sites on the polymer and become entangled in the three-dimensional, polymer structure away from the water.
The chemical formulation of the polymer is a carefully guarded trade secret and a patent application has been made. However, the inventor agreed to share some limited information about the polymer for educational purposes. The hydrocarbon source (crude oil, diesel fuel, gasoline, Mystery Oil, etc.) consists of three basic components: paraffinics, naptinics, and aromatics. The polymer is formulated to specifically bond to these components. The bonding mechanism involves three-dimensional cross-linking so as many as possible are captured. According to the manufacturer, the polymer structures are referred to as dieblock, triblock, branched, radial, and liner.
When the polymer comes in contact with a liquid hydrocarbon, the free hydrocarbons bond to the polymer forming a solid mass. The hydrophobic (water avoiding) properties of the polymer cause it to float on water. However, the structure of the polymer allows it to sink through the hydrocarbon material to maximize its bonding potential. There is no need for mixing since the polymer automatically bonds to free hydrocarbons but not to water.
The polymer is also used in treating oily sludges. It can effectively filter oil field drilling fluids as well. It’s good for stabilizing any other spilled or leaked liquid hydrocarbons that pose a threat to the environment. It’s truly amazing – and timely – chemistry!
It’s obvious that liquid hydrocarbon spills usually occur on water that isn’t at room temperature. You might want to explore how the water temperature could impact the effectiveness of the polymer. What is the best range of water temperature for the most effective cleanup? That might be worked into a very interesting science fair project, too.