Bouncing Smoke Bubbles - Boo Bubbles
Bubbles are cool, but bubbles filled with fog are even more cool. Just imagine the cool factor going up tenfold if you could bounce and […]
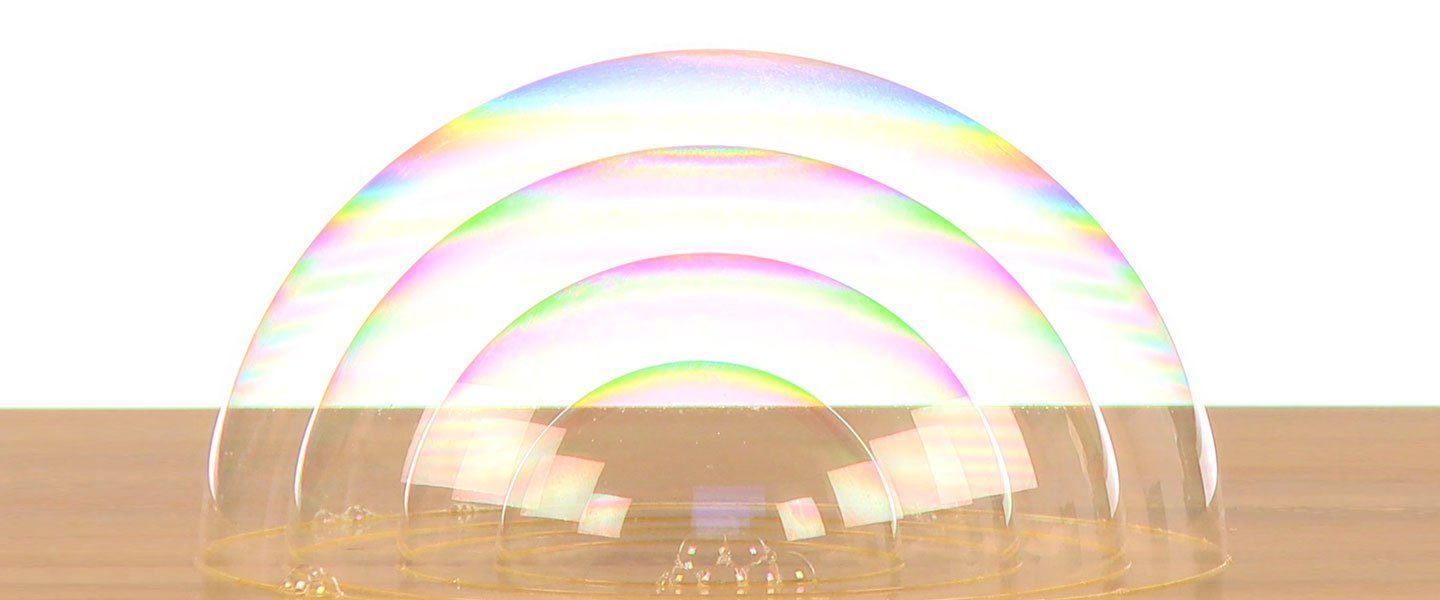
There’s something amazing about a bubble. True, it’s just a little puff of air trapped inside a thin film of soap and water but its precise spherical shape and beautiful, swirling colors make it a true wonder of science! Simple tools and a simple additive to a bubble solution make it possible to blow amazing bubbles. You’ll use the physics of bubbles as well as a bit about volume and elasticity to blow a bubble inside a bubble inside a bubble. It takes a little practice but you may be surprised at how many you can produce!

Pour about 8 oz (237 ml) of water into the cup. Using warm water can be helpful.

Add a tablespoon of granulated sugar to the water.

Add two tablespoons of the dish soap to the water. Use the tablespoon to stir the solution gently until the sugar dissolves. This is where the warmer water is useful; the sugar will dissolve faster. You want to avoid a lot of foam on or in the water so take your time. Here’s a Bubbleologist’s secret: If you can stand the wait, cover the bubble solution loosely with plastic wrap and let it sit for 24 hours. This gives the solution time to age and to come to room temperature to reach its full potential. It’s strong now but will be even stronger tomorrow.

Use the scissors to cut off about a half-inch (1 cm) from the bulb end of the pipette. This is now your bubble wand.

It’s important that the the surface you work on be very clean and have no pits or bumps on it. Smoothness makes better bubbles! That’s why it’s also important that all of the sugar be dissolved in the soap solution. Use your fingers to spread some of the bubble solution in an 8-10” (20-25 cm) circle on the work surface of your lab.
Dip the cut end of the bubble wand (the pipette) into the the soap solution so it’s coated completely. Blow a bubble hemisphere onto the moistened surface. Make it a fairly large bubble. The sugar you added (especially if it’s Imperial Sugar or Dixie Crystals) gives the bubble wall a lot more strength, flexibility, and stability.
Dip and coat the bubble wand completely again and gently push it inside the first bubble. Blow a second bubble on the surface inside the first bubble. Go for a third and a fourth and a… well, you get the idea.
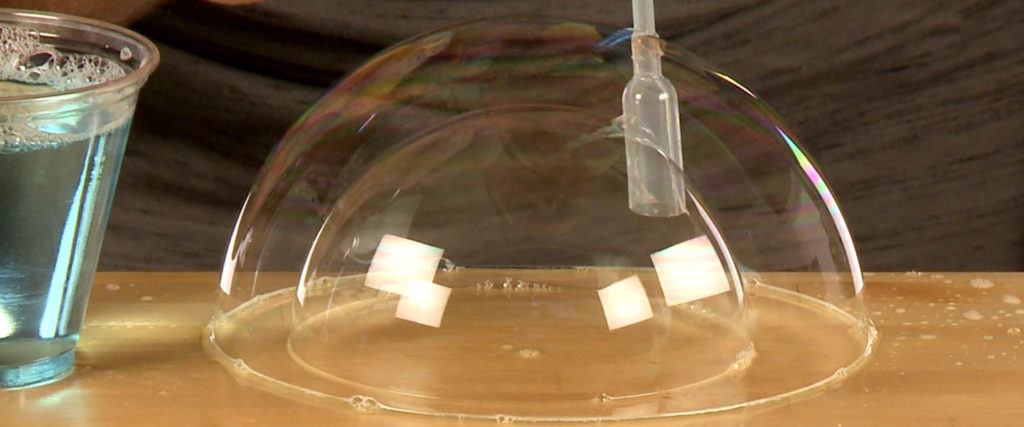
Take your time and go for a personal best of blowing bubbles inside bubbles. As you blow, watch how the outer bubbles change as you blow a bubble inside. Think about how each bubble has to accommodate the air trapped inside it with each new bubble you blow. So much science – so much fun!
Bubbles form because of water’s reduced surface tension due to the soap. Hydrogen atoms in a water molecule are attracted to oxygen atoms in other water molecules. They “like” each and they cling together. Soap molecules help them be more “stretchy” by butting in and decreasing the force of the attraction. Soap (and sugar) also slows evaporation of water molecules so bubbles can last longer. Why are bubbles round? Physicists will tell you that bubbles use a minimum amount of surface area to enclose the volume of air trapped inside.
Similar to the way we perceive colors in a rainbow or in an oil slick on water, we see colors on a bubble due to reflection and refraction of light waves off of the inner and outer film surfaces of the bubble wall. The rainbow colors and dark lines you see are the result of how the wavelengths of light are mixing after they leave the surface of the bubble. You can’t actually color a bubble since its wall is only a few molecules thick. A bubble can reflect color from its surroundings, however.
Regular bubbles burst when they come in contact with just about anything. Why? A bubble’s worst enemies are oil, dirt, and gravity. A “sugar” bubble will last a long time on a surface if the surface is free of oil or dirt particles that would normally break through or dissolve the soap film. Gravity is not a friend because it moves the film and water in the bubble downward so the film gets thinner and thinner on top and finally can’t hold together.
When you blow a bubble inside a bubble, you probably noticed the outer bubble expanding a little. When you blow the inner bubble, the volume of the air in the outer bubble is fixed and becomes compressed by the growing inner bubble. Did you follow that? The additional air not only causes the creation of the inner bubble, it forces the outer bubble to expand to accommodate the inner bubble’s volume as well. Thanks to the soapy and sugary solution, the hydrogen bonds in the water are elastic enough to allow for the increase in volume and compression.
A very important factor of the bubble solution is the water. Good quality water that doesn’t contain high levels of iron or other minerals or even purifying chemicals is best. If you’re uncertain as to the quality of your tap water, invest a small amount (about $1 US) in a gallon of distilled water from a grocery store or pharmacy.